BTN3A molecules considerably improve Vγ9Vδ2T cells-based immunotherapy in acute myeloid leukemia
- PMID: 27853633
- PMCID: PMC5087298
- DOI: 10.1080/2162402X.2016.1146843 (VSports在线直播)
BTN3A molecules considerably improve Vγ9Vδ2T cells-based immunotherapy in acute myeloid leukemia (V体育安卓版)
Abstract
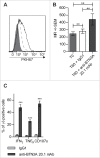
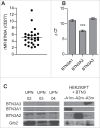
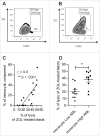
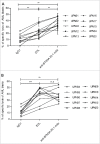
Given their recognized ability to kill acute myeloid leukemia (AML) blasts both in vitro and in vivo, Vγ9Vδ2 T cells are of growing interest in the design of new strategies of immunotherapy. We show that the Butyrophilin3A (BTN3A, CD277) subfamily is a critical determinant of Vγ9Vδ2 TCR-mediated recognition of human primary AML blasts ex vivo. Moreover, anti-BTN3A 20. 1 agonist monoclonal antibodies (mAbs) can trigger BTN3A on AML blasts leading to further enhanced Vγ9Vδ2 T cell-mediated killing, but this mAb had no enhancing effect upon NK cell-mediated killing. We show that monocytic differentiation of primary AML blasts accounts for their AminoBisphosphonate (N-BP)-mediated sensitization to Vγ9Vδ2 T cells. In addition, anti-BTN3A 20. 1 mAbs could specifically sensitize resistant blasts to Vγ9Vδ2 T cells lysis and overcome the poor effect of N-BP treatment on those blasts VSports手机版. We confirmed the enhancement of Vγ9Vδ2 T cells activity by anti-BTN3A 20. 1 mAb using a human AML xenotransplantation mouse model. We showed that anti-BTN3A 20. 1 mAb combined with Vγ9Vδ2 T cells immunotherapy could increase animal survival and decrease the leukemic burden in blood and bone marrow. These findings could be of great interest in the design of new immunotherapeutic strategies for treating AML. .
Keywords: Acute myeloid leukemia; BTN3A; aminobisphosphonate; immunotherapy; monoclonal antibody; γδT cells V体育安卓版. .
Figures


References
-
- Butturini A, Bortin MM, Gale RP. Graft-versus-leukemia following bone marrow transplantation. Bone Marrow Transplant 1987; 2(3):233–42; PMID:3332173 - PubMed
-
- Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000; 96(2):384–92; PMID:10887096; http://dx.doi.org.gate2.inist.fr/ - PubMed
-
- Castella B, Vitale C, Coscia M, Massaia M. Vγ9Vδ2 T cell-based immunotherapy in hematological malignancies: from bench to bedside. Cell Mol Life Sci CMLS 2011; 68(14):2419–32; PMID:21584812; "VSports app下载" http://dx.doi.org/10.1007/s00018-011-0704-8 - DOI - PMC - PubMed
-
- Kabelitz D, Wesch D, Pitters E, Zöller M. Characterization of tumor reactivity of human V gamma 9V delta 2 gamma delta T cells in vitro and in SCID mice in vivo. J Immunol 2004; 173(11):6767–76; PMID:15557170; http://dx.doi.org/10.4049/jimmunol.173.11.6767 - DOI - PubMed
-
- Norell H, Moretta A, Silva-Santos B, Moretta L. At the Bench: Preclinical rationale for exploiting NK cells and γδ T lymphocytes for the treatment of high-risk leukemias. J Leukoc Biol 2013; 94(6):1123–39; PMID:24108703; http://dx.doi.org/10.1189/jlb.0613312 - DOI - PubMed
VSports注册入口 - Publication types
- Actions (VSports)
LinkOut - more resources
"VSports app下载" Full Text Sources
Other Literature Sources
