Listeria monocytogenes-Induced Cell Death Inhibits the Generation of Cell-Mediated Immunity
- PMID: 27821585
- PMCID: PMC5203660
- DOI: "VSports app下载" 10.1128/IAI.00733-16
Listeria monocytogenes-Induced Cell Death Inhibits the Generation of Cell-Mediated Immunity (V体育安卓版)
Abstract
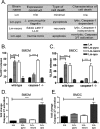
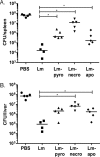
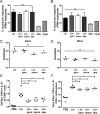
The influence of cell death on adaptive immunity has been studied for decades. Despite these efforts, the intricacies of how various cell death pathways shape immune responses in the context of infection remain unclear, particularly with regard to more recently discovered pathways such as pyroptosis. The emergence of Listeria monocytogenes as a promising immunotherapeutic platform demands a thorough understanding of how cell death induced in the context of infection influences the generation of CD8+ T-cell-mediated immune responses. To begin to address this question, we designed strains of L. monocytogenes that robustly activate necrosis, apoptosis, or pyroptosis VSports手机版. We hypothesized that proinflammatory cell death such as necrosis would be proimmunogenic while apoptosis would be detrimental, as has previously been reported in the context of sterile cell death. Surprisingly, we found that the activation of any host cell death in the context of L. monocytogenes infection inhibited the generation of protective immunity and specifically the activation of antigen-specific CD8+ T cells. Importantly, the mechanism of attenuation was unique for each type of cell death, ranging from deficits in costimulation in the context of necrosis to a suboptimal inflammatory milieu in the case of pyroptosis. Our results suggest that cell death in the context of infection is different from sterile-environment-induced cell death and that inhibition of cell death or its downstream consequences is necessary for developing effective cell-mediated immune responses using L. monocytogenes-based immunotherapeutic platforms. .
Keywords: Listeria monocytogenes; apoptosis; cell death; immunotherapy; inflammasome V体育安卓版. .
Copyright © 2016 American Society for Microbiology. V体育ios版.
Figures




References
-
- Le DT, Dubensky TW, Brockstedt DG. 2012. Clinical development of Listeria monocytogenes-based immunotherapies. Semin Oncol 39:311–322. doi:10.1053/j.seminoncol.2012.02.008. - "VSports手机版" DOI - PMC - PubMed
-
- Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, Uram JN, Laheru DA, Lutz ER, Solt S, Murphy AL, Skoble J, Lemmens E, Grous J, Dubensky T, Brockstedt DG, Jaffee EM. 2015. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 33:1325–1333. doi:10.1200/JCO.2014.57.4244. - DOI - PMC - PubMed
MeSH terms
- Actions (VSports手机版)
- "V体育官网入口" Actions
- "VSports在线直播" Actions
- Actions (V体育ios版)
- "V体育官网入口" Actions
Substances
- VSports注册入口 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

