V体育官网 - Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition
- PMID: 27815355
- PMCID: PMC5413401
- DOI: 10.1158/1078-0432.CCR-16-1300
"V体育官网入口" Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition
"VSports app下载" Abstract
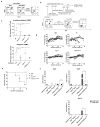
Purpose: Using gene-disrupted allogeneic T cells as universal effector cells provides an alternative and potentially improves current chimeric antigen receptor (CAR) T-cell therapy against cancers and infectious diseases. Experimental Design: The CRISPR/Cas9 system has recently emerged as a simple and efficient way for multiplex genome engineering. By combining lentiviral delivery of CAR and electro-transfer of Cas9 mRNA and gRNAs targeting endogenous TCR, β-2 microglobulin (B2M) and PD1 simultaneously, to generate gene-disrupted allogeneic CAR T cells deficient of TCR, HLA class I molecule and PD1. Results: The CRISPR gene-edited CAR T cells showed potent antitumor activities, both in vitro and in animal models and were as potent as non-gene-edited CAR T cells VSports手机版. In addition, the TCR and HLA class I double deficient T cells had reduced alloreactivity and did not cause graft-versus-host disease. Finally, simultaneous triple genome editing by adding the disruption of PD1 led to enhanced in vivo antitumor activity of the gene-disrupted CAR T cells. Conclusions: Gene-disrupted allogeneic CAR and TCR T cells could provide an alternative as a universal donor to autologous T cells, which carry difficulties and high production costs. Gene-disrupted CAR and TCR T cells with disabled checkpoint molecules may be potent effector cells against cancers and infectious diseases. Clin Cancer Res; 23(9); 2255-66. ©2016 AACR. .
©2016 American Association for Cancer Research V体育安卓版. .
Conflict of interest statement
X V体育ios版. L. , C. H. J. and Y. Z. have financial interests due to intellectual property and patents in the field of cell and gene therapy. Conflicts of interest are managed in accordance with University of Pennsylvania policy and oversight. The other authors declare that they have no competing interests.
Figures (V体育官网入口)




References
-
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science translational medicine. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. - V体育官网 - DOI - PMC - PubMed
-
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. - DOI (VSports) - PMC - PubMed
-
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(7):917–24. doi: 10.1200/JCO.2010.32.2537. - "V体育官网" DOI - PMC - PubMed
MeSH terms
- V体育安卓版 - Actions
- Actions (V体育2025版)
- V体育平台登录 - Actions
- "VSports" Actions
- Actions (V体育安卓版)
- "VSports注册入口" Actions
- VSports在线直播 - Actions
- "VSports app下载" Actions
Substances
- Actions (V体育平台登录)
- "V体育安卓版" Actions
V体育ios版 - Supplementary concepts
- "VSports" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports最新版本" Research Materials
Miscellaneous

