Anoctamin 6 Contributes to Cl- Secretion in Accessory Cholera Enterotoxin (Ace)-stimulated Diarrhea: AN ESSENTIAL ROLE FOR PHOSPHATIDYLINOSITOL 4,5-BISPHOSPHATE (PIP2) SIGNALING IN CHOLERA (VSports)
- PMID: 27799301
- PMCID: "V体育官网" PMC5207189
- DOI: VSports手机版 - 10.1074/jbc.M116.719823
Anoctamin 6 Contributes to Cl- Secretion in Accessory Cholera Enterotoxin (Ace)-stimulated Diarrhea: AN ESSENTIAL ROLE FOR PHOSPHATIDYLINOSITOL 4,5-BISPHOSPHATE (PIP2) SIGNALING IN CHOLERA
Abstract
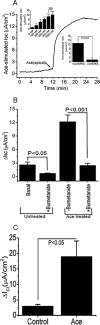
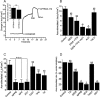
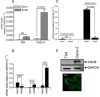
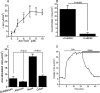
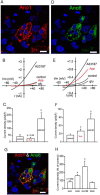
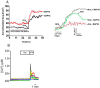
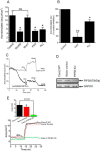
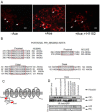
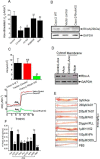
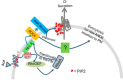
Accessory cholera enterotoxin (Ace) of Vibrio cholerae has been shown to contribute to diarrhea. However, the signaling mechanism and specific type of Cl- channel activated by Ace are still unknown. We have shown here that the recombinant Ace protein induced ICl of apical plasma membrane, which was inhibited by classical CaCC blockers. Surprisingly, an Ace-elicited rise of current was neither affected by ANO1 (TMEM16A)-specific inhibitor T16A(inh)-AO1(TAO1) nor by the cystic fibrosis transmembrane conductance regulator (CFTR) blocker, CFTR inh-172. Ace stimulated whole-cell current in Caco-2 cells. However, the apical ICl was attenuated by knockdown of ANO6 (TMEM16F). This impaired phenotype was restored by re-expression of ANO6 in Caco-2 cells. Whole-cell patch clamp recordings of ANO currents in HEK293 cells transiently expressing mouse ANO1-mCherry or ANO6-GFP confirmed that Ace induced Cl- secretion. Application of Ace produced ANO6 but not the ANO1 currents. Ace was not able to induce a [Ca2+]i rise in Caco-2 cells, but cellular abundance of phosphatidylinositol 4,5-bisphosphate (PIP2) increased. Identification of the PIP2-binding motif at the N-terminal sequence among human and mouse ANO6 variants along with binding of PIP2 directly to ANO6 in HEK293 cells indicate likely PIP2 regulation of ANO6. The biophysical and pharmacological properties of Ace stimulated Cl- current along with intestinal fluid accumulation, and binding of PIP2 to the proximal KR motif of channel proteins, whose mutagenesis correlates with altered binding of PIP2, is comparable with ANO6 stimulation VSports手机版. We conclude that ANO6 is predominantly expressed in intestinal epithelia, where it contributes secretory diarrhea by Ace stimulation in a calcium-independent mechanism of RhoA-ROCK-PIP2 signaling. .
Keywords: epithelial cell; epithelial cell adhesion molecule (EpCAM); intestinal epithelium; ion channel; membrane; pathogenesis; phosphatidylinositol; signaling V体育安卓版. .
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc V体育ios版. .
"VSports在线直播" Figures

V体育ios版 - References
MeSH terms
- "V体育平台登录" Actions
- Actions (V体育官网入口)
- "V体育ios版" Actions
- "VSports最新版本" Actions
- VSports app下载 - Actions
- "V体育平台登录" Actions
- V体育安卓版 - Actions
- "VSports" Actions
- Actions (VSports app下载)
- Actions (VSports)
- Actions (V体育官网)
- "V体育安卓版" Actions
- VSports在线直播 - Actions
- Actions (V体育ios版)
- Actions (V体育官网)
- "V体育ios版" Actions
- "V体育官网入口" Actions
- "VSports注册入口" Actions
- "VSports注册入口" Actions
V体育官网 - Substances
- Actions (VSports注册入口)
- "V体育官网" Actions
- V体育官网 - Actions
- V体育平台登录 - Actions
- "V体育ios版" Actions
- "V体育安卓版" Actions
- "V体育官网" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical (V体育官网)
Miscellaneous

