"V体育2025版" Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps
- PMID: 27798263
- PMCID: PMC5550900
- DOI: "VSports注册入口" 10.1126/scitranslmed.aag1711
Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps
Abstract
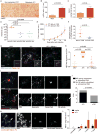
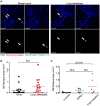
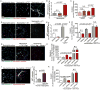
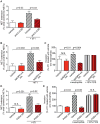
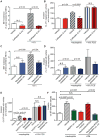
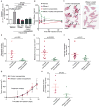
Neutrophils, the most abundant type of leukocytes in blood, can form neutrophil extracellular traps (NETs). These are pathogen-trapping structures generated by expulsion of the neutrophil's DNA with associated proteolytic enzymes. NETs produced by infection can promote cancer metastasis. We show that metastatic breast cancer cells can induce neutrophils to form metastasis-supporting NETs in the absence of infection. Using intravital imaging, we observed NET-like structures around metastatic 4T1 cancer cells that had reached the lungs of mice. We also found NETs in clinical samples of triple-negative human breast cancer. The formation of NETs stimulated the invasion and migration of breast cancer cells in vitro. Inhibiting NET formation or digesting NETs with deoxyribonuclease I (DNase I) blocked these processes. Treatment with NET-digesting, DNase I-coated nanoparticles markedly reduced lung metastases in mice VSports手机版. Our data suggest that induction of NETs by cancer cells is a previously unidentified metastasis-promoting tumor-host interaction and a potential therapeutic target. .
Copyright © 2016, American Association for the Advancement of Science V体育安卓版. .
Figures
"VSports注册入口" Comment in
-
Cancer: Blocking metastasis.Nat Rev Drug Discov. 2016 Dec;15(12):822. doi: 10.1038/nrd.2016.239. Epub 2016 Nov 18. Nat Rev Drug Discov. 2016. PMID: 27857143 No abstract available.
References (VSports手机版)
-
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. - "VSports最新版本" PubMed
-
- Sethi N, Kang Y. Unravelling the complexity of metastasis - molecular understanding and targeted therapies. Nat Rev Cancer. 2011;11:735–748. - "V体育官网入口" PMC - PubMed
-
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. - PubMed
-
- Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221–2232. - PMC - PubMed
Publication types
- "V体育ios版" Actions
VSports在线直播 - MeSH terms
- "V体育ios版" Actions
- VSports手机版 - Actions
- "V体育安卓版" Actions
- Actions (V体育官网)
- "VSports在线直播" Actions
- Actions (VSports app下载)
- "V体育安卓版" Actions
- "VSports" Actions
- "VSports在线直播" Actions
- Actions (V体育官网)
Substances
- "VSports手机版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

