Six3 dosage mediates the pathogenesis of holoprosencephaly
- PMID: 27770010
- PMCID: PMC5201039
- DOI: 10.1242/dev.132142
"V体育2025版" Six3 dosage mediates the pathogenesis of holoprosencephaly
Abstract
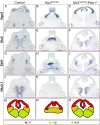
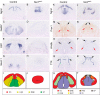
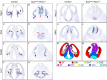
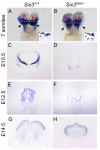
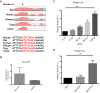
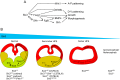
Holoprosencephaly (HPE) is defined as the incomplete separation of the two cerebral hemispheres. The pathology of HPE is variable and, based on the severity of the defect, HPE is divided into alobar, semilobar, and lobar VSports手机版. Using a novel hypomorphic Six3 allele, we demonstrate in mice that variability in Six3 dosage results in different HPE phenotypes. Furthermore, we show that whereas the semilobar phenotype results from severe downregulation of Shh expression in the rostral diencephalon ventral midline, the alobar phenotype is caused by downregulation of Foxg1 expression in the anterior neural ectoderm. Consistent with these results, in vivo activation of the Shh signaling pathway rescued the semilobar phenotype but not the alobar phenotype. Our findings show that variations in Six3 dosage result in different forms of HPE. .
Keywords: Forebrain; Gene dosage; Holoprosencephaly; Mouse; Six3; Transcription factor. V体育安卓版.
© 2016 V体育ios版. Published by The Company of Biologists Ltd. .
V体育安卓版 - Conflict of interest statement
The authors declare no competing or financial interests.
"V体育平台登录" Figures



References
-
- Carl M., Loosli F. and Wittbrodt J. (2002). Six3 inactivation reveals its essential role for the formation and patterning of the vertebrate eye. Development 129, 4057-4063. - PubMed
Publication types
MeSH terms
- Actions (V体育官网入口)
- VSports最新版本 - Actions
- Actions (V体育2025版)
- Actions (V体育平台登录)
- "V体育官网" Actions
- VSports app下载 - Actions
- V体育2025版 - Actions
- V体育平台登录 - Actions
- Actions (VSports)
- Actions (VSports app下载)
- VSports最新版本 - Actions
- Actions (V体育官网)
- "VSports最新版本" Actions
- VSports最新版本 - Actions
Substances
- "V体育安卓版" Actions
- Actions (VSports app下载)
- Actions (V体育安卓版)
- Actions (V体育2025版)
Grants and funding
LinkOut - more resources
Full Text Sources
VSports注册入口 - Other Literature Sources
Molecular Biology Databases

