Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury
- PMID: 27734833
- PMCID: PMC5065628
- DOI: 10.1038/ncomms13096
"VSports手机版" Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury
Abstract
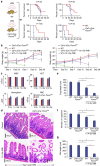
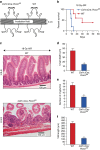
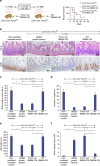
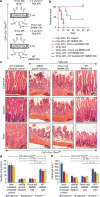
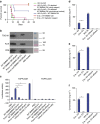
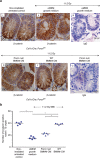
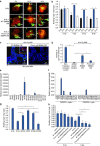
WNT/β-catenin signalling is crucial for intestinal homoeostasis. The intestinal epithelium and stroma are the major source of WNT ligands but their origin and role in intestinal stem cell (ISC) and epithelial repair remains unknown. Macrophages are a major constituent of the intestinal stroma VSports手机版. Here, we analyse the role of macrophage-derived WNT in intestinal repair in mice by inhibiting their release using a macrophage-restricted ablation of Porcupine, a gene essential for WNT synthesis. Such Porcn-depleted mice have normal intestinal morphology but are hypersensitive to radiation injury in the intestine compared with wild-type (WT) littermates. Porcn-null mice are rescued from radiation lethality by treatment with WT but not Porcn-null bone marrow macrophage-conditioned medium (CM). Depletion of extracellular vesicles (EV) from the macrophage CM removes WNT function and its ability to rescue ISCs from radiation lethality. Therefore macrophage-derived EV-packaged WNTs are essential for regenerative response of intestine against radiation. .
Figures
References
-
- Kabiri Z. et al.. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141, 2206–2215 (2014). - PubMed
-
- Saha S. et al.. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS ONE 6, e24072 (2011). - "VSports最新版本" PMC - PubMed
Publication types
MeSH terms
- Actions (V体育ios版)
- V体育2025版 - Actions
- "V体育平台登录" Actions
- Actions (VSports app下载)
- V体育安卓版 - Actions
- V体育官网入口 - Actions
- V体育官网 - Actions
- Actions (VSports注册入口)
- Actions (V体育平台登录)
- V体育安卓版 - Actions
- "VSports最新版本" Actions
- Actions (V体育官网入口)
Substances
- "V体育2025版" Actions
- Actions (VSports在线直播)
- "V体育平台登录" Actions
- Actions (VSports注册入口)
Grants and funding
- P01 CA100324/CA/NCI NIH HHS/United States
- U19 AI091175/AI/NIAID NIH HHS/United States
- MR/N022556/1/MRC_/Medical Research Council/United Kingdom
- "VSports" U01 DK103155/DK/NIDDK NIH HHS/United States
- K12 CA132783/CA/NCI NIH HHS/United States
- G1002033/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- P30 CA013330/CA/NCI NIH HHS/United States
- U24 DK085532/DK/NIDDK NIH HHS/United States
- K01 DK096032/DK/NIDDK NIH HHS/United States
- U01 DK085532/DK/NIDDK NIH HHS/United States
- UL1 TR001073/TR/NCATS NIH HHS/United States
LinkOut - more resources
"V体育安卓版" Full Text Sources
Other Literature Sources (VSports注册入口)
Medical
Molecular Biology Databases

