Cancer Cells with Alternative Lengthening of Telomeres Do Not Display a General Hypersensitivity to ATR Inhibition
- PMID: 27602331
- PMCID: PMC4993795
- DOI: 10.3389/fonc.2016.00186
Cancer Cells with Alternative Lengthening of Telomeres Do Not Display a General Hypersensitivity to ATR Inhibition
Abstract
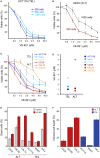
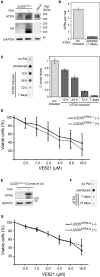
Telomere maintenance is a hallmark of cancer as it provides cancer cells with cellular immortality. A significant fraction of tumors uses the alternative lengthening of telomeres (ALT) pathway to elongate their telomeres and to gain an unlimited proliferation potential. Since the ALT pathway is unique to cancer cells, it represents a potentially valuable, currently unexploited target for anti-cancer therapies. Recently, it was proposed that ALT renders cells hypersensitive to ataxia telangiectasia- and RAD3-related (ATR) protein inhibitors (Flynn et al. , Science 347, 273). Here, we measured the response of various ALT- or telomerase-positive cell lines to the ATR inhibitor VE-821 VSports手机版. In addition, we compared the effect of the inhibitor on cell viability in isogenic cell lines, in which ALT was active or suppressed. In these experiments, a general ATR inhibitor sensitivity of cells with ALT could not be confirmed. We rather propose that the observed variations in sensitivity reflect differences between cell lines that are unrelated to ALT. .
Keywords: ATR inhibitor; VE-821; alternative lengthening of telomeres; ataxia telangiectasia- and RAD3-related V体育安卓版. .
"V体育ios版" Figures
References
-
- Flynn RL, Cox KE, Jeitany M, Wakimoto H, Bryll AR, Ganem NJ, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science (2015) 347(6219):273–7.10.1126/science.1257216 - VSports手机版 - DOI - PMC - PubMed
-
- Asai A, Oshima Y, Yamamoto Y, Uochi TA, Kusaka H, Akinaga S, et al. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res (2003) 63(14):3931–9. - PubMed (V体育ios版)
V体育ios版 - LinkOut - more resources
"V体育平台登录" Full Text Sources
Other Literature Sources
Miscellaneous

