VSports app下载 - Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells
- PMID: 27590511
- PMCID: "V体育平台登录" PMC5323097
- DOI: 10.18632/oncotarget.11743
Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells
Abstract (V体育ios版)
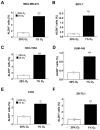
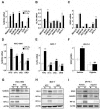
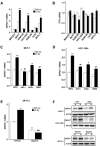
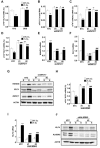
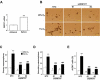
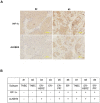
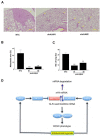
Exposure of breast cancer cells to hypoxia increases the percentage of breast cancer stem cells (BCSCs), which are required for tumor initiation and metastasis, and this response is dependent on the activity of hypoxia-inducible factors (HIFs). We previously reported that exposure of breast cancer cells to hypoxia induces the ALKBH5-mediated demethylation of N6-methyladenosine (m6A) in NANOG mRNA leading to increased expression of NANOG, which is a pluripotency factor that promotes BCSC specification. Here we report that exposure of breast cancer cells to hypoxia also induces ZNF217-dependent inhibition of m6A methylation of mRNAs encoding NANOG and KLF4, which is another pluripotency factor that mediates BCSC specification. Although hypoxia induced the BCSC phenotype in all breast-cancer cell lines analyzed, it did so through variable induction of pluripotency factors and ALKBH5 or ZNF217. However, in every breast cancer line, the hypoxic induction of pluripotency factor and ALKBH5 or ZNF217 expression was HIF-dependent. Immunohistochemistry revealed that expression of HIF-1α and ALKBH5 was concordant in all human breast cancer biopsies analyzed. ALKBH5 knockdown in MDA-MB-231 breast cancer cells significantly decreased metastasis from breast to lungs in immunodeficient mice VSports手机版. Thus, HIFs stimulate pluripotency factor expression and BCSC specification by negative regulation of RNA methylation. .
Keywords: N6-methyladenosine; breast cancer stem cells; hypoxia; metastasis; pluripotency factors V体育安卓版. .
Conflict of interest statement (V体育安卓版)
The authors declare no conflict of interest.
Figures
References
-
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–291. - PubMed
-
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. - PubMed (VSports)
-
- Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106(33):13820–13825. - PMC - PubMed
VSports手机版 - MeSH terms
- "V体育官网" Actions
- V体育官网 - Actions
- V体育平台登录 - Actions
- "VSports注册入口" Actions
- "VSports app下载" Actions
- "V体育安卓版" Actions
- "VSports在线直播" Actions
- "V体育平台登录" Actions
- "V体育安卓版" Actions
- VSports手机版 - Actions
- V体育平台登录 - Actions
- V体育ios版 - Actions
- "VSports手机版" Actions
- "V体育官网入口" Actions
- "V体育ios版" Actions
- V体育2025版 - Actions
Substances
- "V体育安卓版" Actions
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- Actions (VSports)
- VSports在线直播 - Actions
- "V体育平台登录" Actions
- Actions (V体育官网入口)
- Actions (VSports最新版本)
"VSports注册入口" LinkOut - more resources
Full Text Sources
V体育安卓版 - Other Literature Sources
Medical
Research Materials
Miscellaneous (V体育ios版)

