"V体育官网" Enhanced immune response of MAIT cells in tuberculous pleural effusions depends on cytokine signaling
- PMID: 27586092
- PMCID: PMC5009363
- DOI: 10.1038/srep32320
Enhanced immune response of MAIT cells in tuberculous pleural effusions depends on cytokine signaling (V体育平台登录)
Abstract
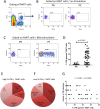
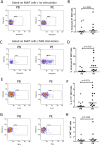
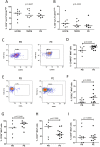
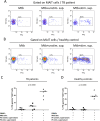
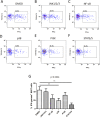
The functions of MAIT cells at the site of Mycobacterium tuberculosis infection in humans are still largely unknown. In this study, the phenotypes and immune response of MAIT cells from tuberculous pleural effusions and peripheral blood were investigated. MAIT cells in tuberculous pleural effusions had greatly enhanced IFN-γ, IL-17F and granzyme B response compared with those in peripheral blood. The level of IFN-γ response in MAIT cells from tuberculous pleural effusions was inversely correlated with the extent of tuberculosis infection (p = 0. 0006). To determine whether cytokines drive the immune responses of MAIT cells at the site of tuberculosis infection, the role of IL-1β, IL-2, IL-7, IL-12, IL-15 and IL-18 was investigated. Blockade of IL-2, IL-12 or IL-18 led to significantly reduced production of IFN-γ and/or granzyme B in MAIT cells from tuberculous pleural effusions VSports手机版. Majority of IL-2-producing cells (94. 50%) in tuberculous pleural effusions had phenotype of CD3(+)CD4(+), and most IL-12p40-producing cells (91. 39%) were CD14(+) cells. MAIT cells had significantly elevated expression of γc receptor which correlated with enhanced immune responses of MAIT cells. It is concluded that MAIT cells from tuberculous pleural effusions exhibited highly elevated immune response to Mtb antigens, which are controlled by cytokines produced by innate/adaptive immune cells. .
Figures
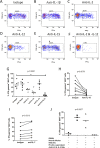
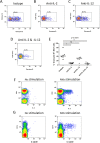
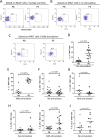
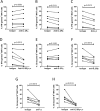
References
-
- WHO. Global tuberculosis report 2014. "VSports最新版本" http://www.who.int/tb/publications/global_report/en/ (2015).
-
- Udwadia Z. F. & Sen T. Pleural tuberculosis: an update. Curr Opin Pulm Med 16, 399–406 (2010). - V体育平台登录 - PubMed
-
- Light R. W. Update on tuberculous pleural effusion. Respirology 15, 451–458 (2010). - PubMed
-
- Le Bourhis L. et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 11, 701–708 (2010). - PubMed
"VSports在线直播" Publication types
- "V体育安卓版" Actions
MeSH terms
- VSports手机版 - Actions
- V体育安卓版 - Actions
- Actions (V体育2025版)
- V体育2025版 - Actions
- V体育ios版 - Actions
- VSports最新版本 - Actions
- V体育官网 - Actions
- V体育安卓版 - Actions
- "V体育官网入口" Actions
- V体育平台登录 - Actions
- Actions (V体育2025版)
- Actions (V体育官网)
- Actions (V体育平台登录)
- "V体育官网入口" Actions
- Actions (VSports注册入口)
- Actions (V体育平台登录)
- "VSports app下载" Actions
"VSports手机版" Substances
- VSports app下载 - Actions
- VSports最新版本 - Actions
- Actions (V体育安卓版)
- "VSports app下载" Actions
- "VSports注册入口" Actions
- VSports在线直播 - Actions
VSports手机版 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials (VSports手机版)
Miscellaneous (VSports在线直播)

