The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke (VSports注册入口)
- PMID: 27585394
- PMCID: PMC5248967
- DOI: 10.1165/rcmb.2016-0226OC
The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke
Abstract
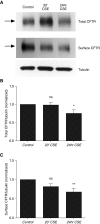
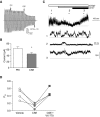
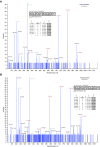
Acquired cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction may contribute to chronic obstructive pulmonary disease pathogenesis and is a potential therapeutic target. We sought to determine the acute effects of cigarette smoke on ion transport and the mucociliary transport apparatus, their mechanistic basis, and whether deleterious effects could be reversed with the CFTR potentiator ivacaftor (VX-770). Primary human bronchial epithelial (HBE) cells and human bronchi were exposed to cigarette smoke extract (CSE) and/or ivacaftor. CFTR function and expression were measured in Ussing chambers and by surface biotinylation. CSE-derived acrolein modifications on CFTR were determined by mass spectroscopic analysis of purified protein, and the functional microanatomy of the airway epithelia was measured by 1-μm resolution optical coherence tomography. CSE reduced CFTR-dependent current in HBE cells (P < 0. 05) and human bronchi (P < 0. 05) within minutes of exposure. The mechanism involved CSE-induced reduction of CFTR gating, decreasing CFTR open-channel probability by approximately 75% immediately after exposure (P < 0. 05), whereas surface CFTR expression was partially reduced with chronic exposure, but was stable acutely. CSE treatment of purified CFTR resulted in acrolein modifications on lysine and cysteine residues that likely disrupt CFTR gating VSports手机版. In primary HBE cells, CSE reduced airway surface liquid depth (P < 0. 05) and ciliary beat frequency (P < 0. 05) within 60 minutes that was restored by coadministration with ivacaftor (P < 0. 005). Cigarette smoking transmits acute reductions in CFTR activity, adversely affecting the airway surface. These effects are reversible by a CFTR potentiator in vitro, representing a potential therapeutic strategy in patients with chronic obstructive pulmonary disease with chronic bronchitis. .
Keywords: cigarette smoke; cystic fibrosis transmembrane conductance regulator potentiator; ivacaftor; mucociliary transport; optical coherence tomography. V体育安卓版.
Figures


References
-
- National Heart, Lung, and Blood Institute. Unpublished Tabulations of the National Health Interview Survey. 2010 [accessed 2016 Aug 20]. Available from: http://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf Data Source http://www.cdc.gov/nchs/nhis/nhis_2010_data_release.htm.
-
- Xu JQ, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: Final data for 2007. National vital statistics reports; vol 58 no 19. Hyattsville, MD: National Center for Health Statistics. 2010 [accessed 2016 Aug 20]. Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_19.pdf.
-
- National Heart, Lung, and Blood Institute. COPD: the more you know, the better for you and your loved ones. Sep 2013 [accessed 2014 Jun 10]. Available from: https://www.nhlbi.nih.gov/health/educational/copd/campaign-materials/pub....
-
- Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288:L894–L902. - "VSports最新版本" PubMed
"VSports注册入口" Publication types
- "V体育安卓版" Actions
MeSH terms
- V体育ios版 - Actions
- Actions (VSports)
- V体育平台登录 - Actions
- "V体育ios版" Actions
- "V体育平台登录" Actions
- VSports注册入口 - Actions
- Actions (VSports在线直播)
- V体育ios版 - Actions
- Actions (V体育ios版)
- Actions (V体育官网入口)
Substances
- Actions (VSports app下载)
Grants and funding
V体育安卓版 - LinkOut - more resources
Full Text Sources
"V体育官网" Other Literature Sources
Medical

