An Integrated Multi-Omic Approach to Assess Radiation Injury on the Host-Microbiome Axis (VSports app下载)
- PMID: 27512828
- PMCID: PMC5304359 (VSports在线直播)
- DOI: 10.1667/RR14306.1 (V体育2025版)
An Integrated Multi-Omic Approach to Assess Radiation Injury on the Host-Microbiome Axis
Abstract
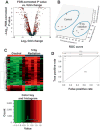
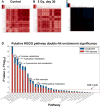
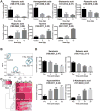
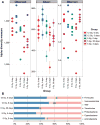
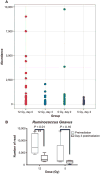
Medical responders to radiological and nuclear disasters currently lack sufficient high-throughput and minimally invasive biodosimetry tools to assess exposure and injury in the affected populations. For this reason, we have focused on developing robust radiation exposure biomarkers in easily accessible biofluids such as urine, serum and feces. While we have previously reported on urine and serum biomarkers, here we assessed perturbations in the fecal metabolome resulting from exposure to external X radiation in vivo. The gastrointestinal (GI) system is of particular importance in radiation biodosimetry due to its constant cell renewal and sensitivity to radiation-induced injury. While the clinical GI symptoms such as pain, bloating, nausea, vomiting and diarrhea are manifested after radiation exposure, no reliable bioindicator has been identified for radiation-induced gastrointestinal injuries. To this end, we focused on determining a fecal metabolomic signature in X-ray irradiated mice. There is overwhelming evidence that the gut microbiota play an essential role in gut homeostasis and overall health VSports手机版. Because the fecal metabolome is tightly correlated with the composition and diversity of the microorganism in the gut, we also performed fecal 16S rRNA sequencing analysis to determine the changes in the microbial composition postirradiation. We used in-house bioinformatics tools to integrate the 16S rRNA sequencing and metabolomic data, and to elucidate the gut integrated ecosystem and its deviations from a stable host-microbiome state that result from irradiation. The 16S rRNA sequencing results indicated that radiation caused remarkable alterations of the microbiome in feces at the family level. Increased abundance of common members of Lactobacillaceae and Staphylococcaceae families, and decreased abundances of Lachnospiraceae, Ruminococcaceae and Clostridiaceae families were found after 5 and 12 Gy irradiation. The metabolomic data revealed statistically significant changes in the microbial-derived products such as pipecolic acid, glutaconic acid, urobilinogen and homogentisic acid. In addition, significant changes were detected in bile acids such as taurocholic acid and 12-ketodeoxycholic acid. These changes may be associated with the observed shifts in the abundance of intestinal microbes, such as R. gnavus , which can transform bile acids. .
Figures
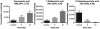

References
-
- Asselin C, Gendron FP. Shuttling of information between the mucosal and luminal environment drives intestinal homeostasis. FEBS Lett. 2014;588:4148–57. - PubMed
-
- McLaughlin MM, Dacquisto MP, Jacobus DP, Horowitz RE. Effects of the germ free state on responses of mice to whole-body irradiation. Radiat Res. 1964;23:333–49. - VSports - PubMed
-
- Sartor RB. Microbial-host interactions in inflammatory bowel diseases and experimental colitis. Nestle Nutr Workshop Ser Pediatr Program. 2009;64:121–32. - PubMed
-
- Polistena A, Johnson LB, Ohiami-Masseron S, Wittgren L, Bäck S, Thornberg C, et al. Local radiotherapy of exposed murine small bowel: apoptosis and inflammation. BMC Surg. 2008;8:1. - "V体育官网" PMC - PubMed
-
- Nejdfors P, Ekelund M, Westrom BR, Willen R, Jeppsson B. Intestinal permeability in humans is increased after radiation therapy. Dis Colon Rectum. 2000;43:1582–7. - PubMed
Publication types (VSports app下载)
- "VSports" Actions
MeSH terms (VSports app下载)
- "V体育平台登录" Actions
- V体育ios版 - Actions
- "V体育2025版" Actions
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

