Sex-specific differences in neonatal hyperoxic lung injury
- PMID: 27343189
- PMCID: PMC5504425 (V体育平台登录)
- DOI: 10.1152/ajplung.00047.2016
Sex-specific differences in neonatal hyperoxic lung injury
Abstract
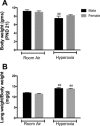
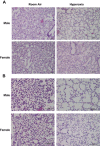
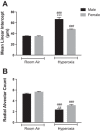
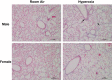
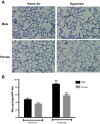
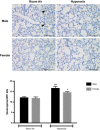
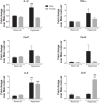
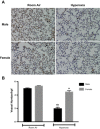
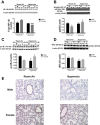
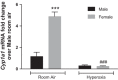
Male sex is considered an independent predictor for the development of bronchopulmonary dysplasia (BPD) after adjusting for other confounders. BPD is characterized by an arrest in lung development with marked impairment of alveolar septation and vascular development. The reasons underlying sexually dimorphic outcomes in premature neonates are not known. In this investigation, we tested the hypothesis that male neonatal mice will be more susceptible to hyperoxic lung injury and will display larger arrest in lung alveolarization. Neonatal male and female mice (C57BL/6) were exposed to hyperoxia [95% FiO2, postnatal day (PND) 1-5] and euthanized on PND 7 and 21. Extent of alveolarization, pulmonary vascularization, inflammation, and modulation of the NF-κB pathway were determined and compared with room air controls. Macrophage and neutrophil infiltration was significantly increased in hyperoxia-exposed animals but was increased to a larger extent in males compared with females. Lung morphometry showed a higher mean linear intercept (MLI) and a lower radial alveolar count (RAC) and therefore greater arrest in lung development in male mice. This was accompanied by a significant decrease in the expression of markers of angiogenesis (PECAM1 and VEGFR2) in males after hyperoxia exposure compared with females. Interestingly, female mice showed increased activation of the NF-κB pathway in the lungs compared with males VSports手机版. These results support the hypothesis that sex plays a crucial role in hyperoxia-mediated lung injury in this model. Elucidation of the sex-specific molecular mechanisms may aid in the development of novel individualized therapies to prevent/treat BPD. .
Keywords: bronchopulmonary dysplasia; gender; hyperoxia; lung development; sex. V体育安卓版.
Copyright © 2016 the American Physiological Society. V体育ios版.
Figures

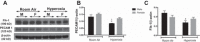
References
-
- Ambalavanan N, Carlo WA, McDonald SA, Yao Q, Das A, Higgins RD. Generic Database, and Follow-up Subcommittees of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Identification of extremely premature infants at high risk of rehospitalization. Pediatrics 128: e1216–e1225, 2011. - "VSports app下载" PMC - PubMed
-
- Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. I. The term mouse models. Am J Physiol Lung Cell Mol Physiol 307: L936–L947, 2014. - PMC (V体育官网) - PubMed
-
- Bhandari V. Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med 15: 223–229, 2010. - V体育2025版 - PMC - PubMed
-
- Binet ME, Bujold E, Lefebvre F, Tremblay Y, Piedboeuf B. Canadian Neonatal Network. Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol 29: 159–166, 2012. - PubMed
-
- Bolon B. Pathology of the Developing Mouse. Boca Raton, FL: CRC, 2015.
V体育官网 - Publication types
MeSH terms
- "V体育2025版" Actions
- Actions (V体育安卓版)
- V体育ios版 - Actions
- "V体育安卓版" Actions
- "V体育ios版" Actions
V体育官网 - Substances
- "VSports在线直播" Actions
V体育ios版 - Grants and funding
"V体育2025版" LinkOut - more resources
"VSports app下载" Full Text Sources
"VSports在线直播" Other Literature Sources
Miscellaneous

