Cathepsin Protease Controls Copper and Cisplatin Accumulation via Cleavage of the Ctr1 Metal-binding Ectodomain
- PMID: 27143361
- PMCID: PMC4933151
- DOI: VSports最新版本 - 10.1074/jbc.M116.731281
Cathepsin Protease Controls Copper and Cisplatin Accumulation via Cleavage of the Ctr1 Metal-binding Ectodomain
Abstract
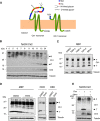
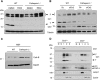
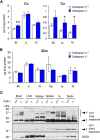
Copper is an essential metal ion for embryonic development, iron acquisition, cardiac function, neuropeptide biogenesis, and other critical physiological processes. Ctr1 is a high affinity Cu(+) transporter on the plasma membrane and endosomes that exists as a full-length protein and a truncated form of Ctr1 lacking the methionine- and histidine-rich metal-binding ectodomain, and it exhibits reduced Cu(+) transport activity. Here, we identify the cathepsin L/B endolysosomal proteases functioning in a direct and rate-limiting step in the Ctr1 ectodomain cleavage. Cells and mice lacking cathepsin L accumulate full-length Ctr1 and hyper-accumulate copper VSports手机版. As Ctr1 also transports the chemotherapeutic drug cisplatin via direct binding to the ectodomain, we demonstrate that the combination of cisplatin with a cathepsin L/B inhibitor enhances cisplatin uptake and cell killing. These studies identify a new processing event and the key protease that cleaves the Ctr1 metal-binding ectodomain, which functions to regulate cellular Cu(+) and cisplatin acquisition. .
Keywords: anti-cancer drug; cathepsin; cisplatin; copper transport; cysteine protease; intracellular processing; metal homeostasis; protein processing V体育安卓版. .
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc. V体育ios版.
Figures



References
-
- Kim B. E., Nevitt T., and Thiele D. J. (2008) Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 4, 176–185 - PubMed
-
- Madsen E., and Gitlin J. D. (2007) Copper and iron disorders of the brain. Annu. Rev. Neurosci. 30, 317–337 - PubMed
-
- Medeiros D. M., Davidson J., and Jenkins J. E. (1993) A unified perspective on copper deficiency and cardiomyopathy. Proc. Soc. Exp. Biol. Med. 203, 262–273 - PubMed (V体育2025版)
V体育ios版 - Publication types
- VSports在线直播 - Actions
- Actions (V体育ios版)
MeSH terms
- V体育平台登录 - Actions
- Actions (VSports手机版)
- Actions (V体育安卓版)
- "VSports手机版" Actions
Substances
- VSports - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
V体育ios版 - Research Materials

