"V体育官网入口" Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer
- PMID: 27133165
- PMCID: PMC4874853
- DOI: 10.1016/j.cell.2016.04.009
Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer
Abstract
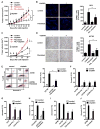
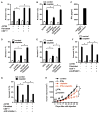
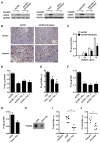
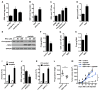
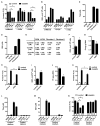
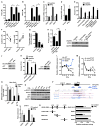
Effector T cells and fibroblasts are major components in the tumor microenvironment VSports手机版. The means through which these cellular interactions affect chemoresistance is unclear. Here, we show that fibroblasts diminish nuclear accumulation of platinum in ovarian cancer cells, resulting in resistance to platinum-based chemotherapy. We demonstrate that glutathione and cysteine released by fibroblasts contribute to this resistance. CD8(+) T cells abolish the resistance by altering glutathione and cystine metabolism in fibroblasts. CD8(+) T-cell-derived interferon (IFN)γ controls fibroblast glutathione and cysteine through upregulation of gamma-glutamyltransferases and transcriptional repression of system xc(-) cystine and glutamate antiporter via the JAK/STAT1 pathway. The presence of stromal fibroblasts and CD8(+) T cells is negatively and positively associated with ovarian cancer patient survival, respectively. Thus, our work uncovers a mode of action for effector T cells: they abrogate stromal-mediated chemoresistance. Capitalizing upon the interplay between chemotherapy and immunotherapy holds high potential for cancer treatment. .
Copyright © 2016 Elsevier Inc. All rights reserved V体育安卓版. .
Figures

Comment in
-
VSports在线直播 - Gynaecological cancer: Chemoresistance - a little help from friends.Nat Rev Clin Oncol. 2016 May 20;13(6):329. doi: 10.1038/nrclinonc.2016.82. Nat Rev Clin Oncol. 2016. PMID: 27199177 No abstract available.
-
Microenvironmental InterFereNce of metabolism regulates chemosensitivity.Cell Res. 2016 Aug;26(8):867-8. doi: 10.1038/cr.2016.82. Epub 2016 Jun 28. Cell Res. 2016. PMID: 27349476 Free PMC article.
References
-
- Binder DC, Fu YX, Weichselbaum RR. Radiotherapy and immune checkpoint blockade: potential interactions and future directions. Trends Mol Med. 2015;21:463–465. - PubMed
-
- Chen HH, Kuo MT. Role of glutathione in the regulation of Cisplatin resistance in cancer chemotherapy. Met Based Drugs. 2010;2010:430939. - "V体育安卓版" PMC - PubMed
-
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. - PubMed
-
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. - PubMed
Publication types
MeSH terms
- Actions (V体育ios版)
- VSports注册入口 - Actions
- Actions (VSports最新版本)
- Actions (V体育2025版)
- VSports app下载 - Actions
- Actions (V体育平台登录)
- "VSports手机版" Actions
Substances
- Actions (VSports手机版)
Grants and funding
LinkOut - more resources (V体育2025版)
Full Text Sources
VSports最新版本 - Other Literature Sources
Medical
Research Materials
Miscellaneous (VSports)

