Mst1 shuts off cytosolic antiviral defense through IRF3 phosphorylation
- PMID: 27125670
- PMCID: PMC4863739
- DOI: 10.1101/gad.277533.116
Mst1 shuts off cytosolic antiviral defense through IRF3 phosphorylation
VSports app下载 - Abstract
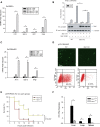
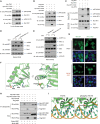
Cytosolic RNA/DNA sensing elicits primary defense against viral pathogens. Interferon regulatory factor 3 (IRF3), a key signal mediator/transcriptional factor of the antiviral-sensing pathway, is indispensible for interferon production and antiviral defense. However, how the status of IRF3 activation is controlled remains elusive. Through a functional screen of the human kinome, we found that mammalian sterile 20-like kinase 1 (Mst1), but not Mst2, profoundly inhibited cytosolic nucleic acid sensing. Mst1 associated with IRF3 and directly phosphorylated IRF3 at Thr75 and Thr253. This Mst1-mediated phosphorylation abolished activated IRF3 homodimerization, its occupancy on chromatin, and subsequent IRF3-mediated transcriptional responses. In addition, Mst1 also impeded virus-induced activation of TANK-binding kinase 1 (TBK1), further attenuating IRF3 activation. As a result, Mst1 depletion or ablation enabled an enhanced antiviral response and defense in cells and mice. Therefore, the identification of Mst1 as a novel physiological negative regulator of IRF3 activation provides mechanistic insights into innate antiviral defense and potential antiviral prevention strategies. VSports手机版.
Keywords: IRF3; Mst1; TBK1; host antiviral defense; phosphorylation. V体育安卓版.
© 2016 Meng et al. ; Published by Cold Spring Harbor Laboratory Press. V体育ios版.
Figures



References
-
- Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124: 783–801. - PubMed
-
- Beis D, Stainier DY. 2006. In vivo cell biology: following the zebrafish trend. Trends Cell Biol 16: 105–112. - PubMed
-
- Callus BA, Verhagen AM, Vaux DL. 2006. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J 273: 4264–4276. - V体育官网 - PubMed
-
- Chae JS, Gil Hwang S, Lim DS, Choi EJ. 2012. Thioredoxin-1 functions as a molecular switch regulating the oxidative stress-induced activation of MST1. Free Radic Biol Med 53: 2335–2343. - PubMed
MeSH terms
- V体育ios版 - Actions
- Actions (VSports在线直播)
- "VSports app下载" Actions
- "VSports最新版本" Actions
- V体育平台登录 - Actions
- V体育2025版 - Actions
- V体育安卓版 - Actions
- V体育安卓版 - Actions
- V体育官网 - Actions
- Actions (V体育2025版)
- V体育官网入口 - Actions
- V体育官网入口 - Actions
"VSports" Substances
- V体育安卓版 - Actions
- "V体育官网" Actions
- "VSports app下载" Actions
- V体育官网 - Actions
- V体育官网入口 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous (V体育2025版)
