Effect of the Spiroiminodihydantoin Lesion on Nucleosome Stability and Positioning
- PMID: 27074396
- PMCID: PMC6299323
- DOI: V体育安卓版 - 10.1021/acs.biochem.6b00093
VSports - Effect of the Spiroiminodihydantoin Lesion on Nucleosome Stability and Positioning
Abstract
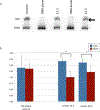
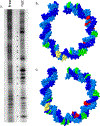
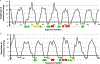
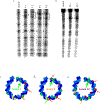
DNA is constantly under attack by oxidants, generating a variety of potentially mutagenic covalently modified species, including oxidized guanine base products. One such product is spiroiminodihydantoin (Sp), a chiral, propeller-shaped lesion that strongly destabilizes the DNA helix in its vicinity VSports手机版. Despite its unusual shape and thermodynamic effect on double-stranded DNA structure, DNA duplexes containing the Sp lesion form stable nucleosomes upon being incubated with histone octamers. Indeed, among six different combinations of lesion location and stereochemistry, only two duplexes display a diminished ability to form nucleosomes, and these only by ∼25%; the other four are statistically indistinguishable from the control. Nonetheless, kinetic factors also play a role: when the histone proteins have less time during assembly of the core particle to sample both lesion-containing and normal DNA strands, they are more likely to bind the Sp lesion DNA than during slower assembly processes that better approximate thermodynamic equilibrium. Using DNase I footprinting and molecular modeling, we discovered that the Sp lesion causes only a small perturbation (±1-2 bp) on the translational position of the DNA within the nucleosome. Each diastereomeric pair of lesions has the same effect on nucleosome positioning, but lesions placed at different locations behave differently, illustrating that the location of the lesion and not its shape serves as the primary determinant of the most stable DNA orientation. .
Figures


References
-
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, and Ellenberger T (2006) DNA Repair and Mutagenesis 2nd ed. ASM Press, Washington, D.C.
-
- Dedon PC (2011) Oxidation and deamination of DNA by endogenous sources In Chemical Carcinogenesis: Current Cancer Research (Penning TM, Ed.) pp. 209–225, Springer Science and Business Media.
-
- Evans MD, Dizdaroglu M, and Cooke MS (2004) Oxidative DNA damage and disease: induction, repair and significance. Mutat. Res. 567, 1–61. - PubMed
-
- Steenken S, and Jovanovic S (1997) How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 119, 617–618.
Publication types
- VSports手机版 - Actions
MeSH terms
- "V体育安卓版" Actions
- Actions (V体育官网)
- "V体育安卓版" Actions
- Actions (V体育安卓版)
- VSports在线直播 - Actions
- "V体育ios版" Actions
Substances
- VSports - Actions
- Actions (VSports)
Grants and funding
LinkOut - more resources
Full Text Sources (VSports注册入口)
Other Literature Sources

