Nonhomologous end joining of complex DNA double-strand breaks with proximal thymine glycol and interplay with base excision repair
- PMID: 27049455
- PMCID: PMC4851560 (V体育ios版)
- DOI: "V体育官网" 10.1016/j.dnarep.2016.03.003
Nonhomologous end joining of complex DNA double-strand breaks with proximal thymine glycol and interplay with base excision repair
Abstract (V体育安卓版)
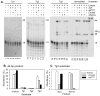
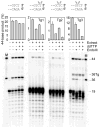
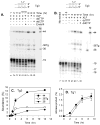
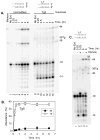
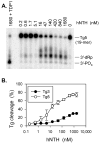
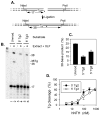
DNA double-strand breaks induced by ionizing radiation are often accompanied by ancillary oxidative base damage that may prevent or delay their repair. In order to better define the features that make some DSBs repair-resistant, XLF-dependent nonhomologous end joining of blunt-ended DSB substrates having the oxidatively modified nonplanar base thymine glycol at the first (Tg1), second (Tg2), third (Tg3) or fifth (Tg5) positions from one 3' terminus, was examined in human whole-cell extracts. Tg at the third position had little effect on end-joining even when present on both ends of the break. However, Tg as the terminal or penultimate base was a major barrier to end joining (>10-fold reduction in ligated products) and an absolute barrier when present at both ends VSports手机版. Dideoxy trapping of base excision repair intermediates indicated that Tg was excised from Tg1, Tg2 and Tg3 largely if not exclusively after DSB ligation. However, Tg was rapidly excised from the Tg5 substrate, resulting in a reduced level of DSB ligation, as well as slow concomitant resection of the opposite strand. Ligase reactions containing only purified Ku, XRCC4, ligase IV and XLF showed that ligation of Tg3 and Tg5 was efficient and only partially XLF-dependent, whereas ligation of Tg1 and Tg2 was inefficient and only detectable in the presence of XLF. Overall, the results suggest that promoting ligation of DSBs with proximal base damage may be an important function of XLF, but that Tg can still be a major impediment to repair, being relatively resistant to both trimming and ligation. Moreover, it appears that base excision repair of Tg can sometimes interfere with repair of DSBs that would otherwise be readily rejoined. .
Copyright © 2016 Elsevier B. V. All rights reserved V体育安卓版. .
Figures



References
-
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist Wolters Kluwer/Lippincott, Williams & Wilkins. 2012.
-
- Ward JF. Biochemistry of DNA lesions. Radiat Res Suppl. 1985;8:S103–11. - PubMed
-
- Goodhead DT. Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA. Int J Radiat Biol. 1994;65:7–17. - PubMed
-
- Georgakilas AG, O’Neill P, Stewart RD. Induction and repair of clustered DNA lesions: What do we know so far? Radiat Res. 2013;180:100–109. - PubMed
Publication types
- "VSports最新版本" Actions
"VSports" MeSH terms
- "VSports最新版本" Actions
- VSports app下载 - Actions
- V体育官网 - Actions
- VSports app下载 - Actions
Substances
- V体育平台登录 - Actions
VSports注册入口 - Grants and funding
LinkOut - more resources
Full Text Sources
V体育官网 - Other Literature Sources
Miscellaneous

