Human MAIT-cell responses to Escherichia coli: activation, cytokine production, proliferation, and cytotoxicity
- PMID: 27034405
- PMCID: PMC4946616
- DOI: 10.1189/jlb.4TA0815-391RR
"V体育安卓版" Human MAIT-cell responses to Escherichia coli: activation, cytokine production, proliferation, and cytotoxicity
Abstract
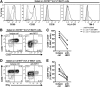
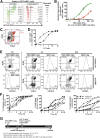
Mucosa-associated invariant T cells are a large and relatively recently described innate-like antimicrobial T-cell subset in humans. These cells recognize riboflavin metabolites from a range of microbes presented by evolutionarily conserved major histocompatibility complex, class I-related molecules. Given the innate-like characteristics of mucosa-associated invariant T cells and the novel type of antigens they recognize, new methodology must be developed and existing methods refined to allow comprehensive studies of their role in human immune defense against microbial infection. In this study, we established protocols to examine a range of mucosa-associated invariant T-cell functions as they respond to antigen produced by Escherichia coli These improved and dose- and time-optimized experimental protocols allow detailed studies of MR1-dependent mucosa-associated invariant T-cell responses to Escherichia coli pulsed antigen-presenting cells, as assessed by expression of activation markers and cytokines, by proliferation, and by induction of apoptosis and death in major histocompatibility complex, class I-related-expressing target cells. The novel and optimized protocols establish a framework of methods and open new possibilities to study mucosa-associated invariant T-cell immunobiology, using Escherichia coli as a model antigen VSports手机版. Furthermore, we propose that these robust experimental systems can also be adapted to study mucosa-associated invariant T-cell responses to other microbes and types of antigen-presenting cells. .
Keywords: MR1; antimicrobial activity; functional evaluation; innate-like T cells V体育安卓版. .
© The Author(s).
Figures


V体育官网入口 - References
-
- Le Bourhis L., Mburu Y. K., Lantz O. (2013) MAIT cells, surveyors of a new class of antigen: development and functions. Curr. Opin. Immunol. 25, 174–180. - PubMed
-
- Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F., Affaticati P., Gilfillan S., Lantz O. (2003) Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169. - PubMed
-
- Leeansyah E., Ganesh A., Quigley M. F., Sönnerborg A., Andersson J., Hunt P. W., Somsouk M., Deeks S. G., Martin J. N., Moll M., Shacklett B. L., Sandberg J. K. (2013) Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 121, 1124–1135. - PMC - PubMed
-
- Walker L. J., Kang Y. H., Smith M. O., Tharmalingham H., Ramamurthy N., Fleming V. M., Sahgal N., Leslie A., Oo Y., Geremia A., Scriba T. J., Hanekom W. A., Lauer G. M., Lantz O., Adams D. H., Powrie F., Barnes E., Klenerman P. (2012) Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood 119, 422–433. - PMC - PubMed
MeSH terms
- "V体育安卓版" Actions
- Actions (VSports手机版)
- Actions (V体育官网入口)
- Actions (VSports最新版本)
- "VSports最新版本" Actions
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- Actions (V体育官网入口)
- V体育官网入口 - Actions
- Actions (V体育官网入口)
VSports app下载 - Substances
- "V体育ios版" Actions
Grants and funding
V体育平台登录 - LinkOut - more resources
Full Text Sources (V体育2025版)
V体育官网 - Other Literature Sources
Medical

