Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis
- PMID: 26964500
- PMCID: PMC4793047
- DOI: 10.1038/ncomms10973
Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis (V体育2025版)
Abstract
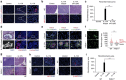
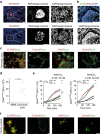
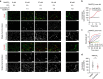
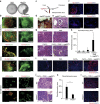
Ductal occlusion has been postulated to precipitate focal pancreatic inflammation, while the nature of the primary occluding agents has remained elusive. Neutrophils make use of histone citrullination by peptidyl arginine deiminase-4 (PADI4) in contact to particulate agents to extrude decondensed chromatin as neutrophil extracellular traps (NETs). In high cellular density, NETs form macroscopically visible aggregates. Here we show that such aggregates form inside pancreatic ducts in humans and mice occluding pancreatic ducts and thereby driving pancreatic inflammation VSports手机版. Experimental models indicate that PADI4 is critical for intraductal aggregate formation and that PADI4-deficiency abrogates disease progression. Mechanistically, we identify the pancreatic juice as a strong instigator of neutrophil chromatin extrusion. Characteristic single components of pancreatic juice, such as bicarbonate ions and calcium carbonate crystals, induce aggregated NET formation. Ductal occlusion by aggregated NETs emerges as a pathomechanism with relevance in a plethora of inflammatory conditions involving secretory ducts. .
Figures


Comment in
-
Pancreatitis: NETs clog pancreatic ducts.Nat Rev Gastroenterol Hepatol. 2016 May;13(5):252. doi: 10.1038/nrgastro.2016.57. Epub 2016 Apr 1. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27033127 No abstract available.
"VSports app下载" References
-
- Lankisch P. G., Apte M. & Banks P. A. Acute pancreatitis. Lancet 386, 85–96 (2015) . - "VSports" PubMed
-
- Lerch M. M. et al.. Advances in the etiology of chronic pancreatitis. Dig. Dis. 28, 324–329 (2010) . - PubMed
-
- Guy O., Robles-Diaz G., Adrich Z., Sahel J. & Sarles H. Protein content of precipitates present in pancreatic juice of alcoholic subjects and patients with chronic calcifying pancreatitis. Gastroenterology 84, 102–107 (1983) . - PubMed (VSports最新版本)
-
- Haruta I. et al.. A mouse model of autoimmune pancreatitis with salivary gland involvement triggered by innate immunity via persistent exposure to avirulent bacteria. Lab. Invest. 90, 1757–1769 (2010) . - "VSports在线直播" PubMed
VSports最新版本 - Publication types
- Actions (V体育ios版)
- "VSports在线直播" Actions
MeSH terms
- Actions (V体育官网)
- "VSports在线直播" Actions
- Actions (VSports手机版)
- "V体育官网入口" Actions
- V体育安卓版 - Actions
- "V体育安卓版" Actions
- "V体育2025版" Actions
- "VSports注册入口" Actions
- "V体育官网" Actions
- VSports最新版本 - Actions
- VSports在线直播 - Actions
Substances
- "VSports最新版本" Actions
- Actions (VSports在线直播)
- "V体育安卓版" Actions
- VSports手机版 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

