Activation of Myenteric Glia during Acute Inflammation In Vitro and In Vivo
- PMID: 26964064
- PMCID: PMC4786261
- DOI: 10.1371/journal.pone.0151335 (V体育官网)
Activation of Myenteric Glia during Acute Inflammation In Vitro and In Vivo
Abstract
Background: Enteric glial cells (EGCs) are the main constituent of the enteric nervous system and share similarities with astrocytes from the central nervous system including their reactivity to an inflammatory microenvironment. Previous studies on EGC pathophysiology have specifically focused on mucosal glia activation and its contribution to mucosal inflammatory processes observed in the gut of inflammatory bowel disease (IBD) patients VSports手机版. In contrast knowledge is scarce on intestinal inflammation not locally restricted to the mucosa but systemically affecting the intestine and its effect on the overall EGC network. .
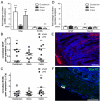
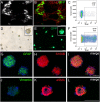
Methods and results: In this study, we analyzed the biological effects of a systemic LPS-induced hyperinflammatory insult on overall EGCs in a rat model in vivo, mimicking the clinical situation of systemic inflammation response syndrome (SIRS). Tissues from small and large intestine were removed 4 hours after systemic LPS-injection and analyzed on transcript and protein level. Laser capture microdissection was performed to study plexus-specific gene expression alterations V体育安卓版. Upon systemic LPS-injection in vivo we observed a rapid and dramatic activation of Glial Fibrillary Acidic Protein (GFAP)-expressing glia on mRNA level, locally restricted to the myenteric plexus. To study the specific role of the GFAP subpopulation, we established flow cytometry-purified primary glial cell cultures from GFAP promotor-driven EGFP reporter mice. After LPS stimulation, we analyzed cytokine secretion and global gene expression profiles, which were finally implemented in a bioinformatic comparative transcriptome analysis. Enriched GFAP+ glial cells cultured as gliospheres secreted increased levels of prominent inflammatory cytokines upon LPS stimulation. Additionally, a shift in myenteric glial gene expression profile was induced that predominantly affected genes associated with immune response. .
Conclusion and significance: Our findings identify the myenteric GFAP-expressing glial subpopulation as particularly susceptible and responsive to acute systemic inflammation of the gut wall and complement knowledge on glial involvement in mucosal inflammation of the intestine. V体育ios版.
Conflict of interest statement
Figures




"V体育平台登录" References
-
- Neunlist M, Aubert P, Bonnaud S, van Landeghem L, Coron E, Wedel T et al. (2007) Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 292 (1): G231–41. - PubMed
-
- Bohórquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, Liddle RA et al. (2014) An Enteroendocrine Cell—Enteric Glia Connection Revealed by 3D Electron Microscopy. PLoS ONE 9 (2): e89881 10.1371/journal.pone.0089881 - "V体育ios版" DOI - PMC - PubMed
-
- Hanani M, Reichenbach A (1994) Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res 278 (1): 153–160. - PubMed
-
- Rühl A (2005) Glial cells in the gut. Neurogastroenterology & Motility 17 (6): 777–790. - PubMed
Publication types
- "V体育官网" Actions
MeSH terms
- "VSports手机版" Actions
- "V体育ios版" Actions
- "V体育平台登录" Actions
- Actions (V体育2025版)
- V体育平台登录 - Actions
- "VSports" Actions
Substances
- "V体育平台登录" Actions
- "VSports在线直播" Actions
VSports app下载 - LinkOut - more resources
Full Text Sources
V体育官网 - Other Literature Sources
"VSports在线直播" Molecular Biology Databases
Miscellaneous

