Cancer therapy in the necroptosis era
- PMID: 26915291
- PMCID: VSports最新版本 - PMC4832112
- DOI: 10.1038/cdd.2016.8
Cancer therapy in the necroptosis era
Abstract
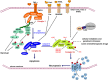
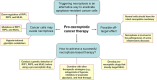
Necroptosis is a caspase-independent form of regulated cell death executed by the receptor-interacting protein kinase 1 (RIP1), RIP3, and mixed lineage kinase domain-like protein (MLKL). Recently, necroptosis-based cancer therapy has been proposed to be a novel strategy for antitumor treatment. However, a big controversy exists on whether this type of therapy is feasible or just a conceptual model. Proponents believe that because necroptosis and apoptosis use distinct molecular pathways, triggering necroptosis could be an alternative way to eradicate apoptosis-resistant cancer cells. This hypothesis has been preliminarily validated by recent studies. However, some skeptics doubt this strategy because of the intrinsic or acquired defects of necroptotic machinery observed in many cancer cells. Moreover, two other concerns are whether or not necroptosis inducers are selective in killing cancer cells without disturbing the normal cells and whether it will lead to inflammatory diseases. In this review, we summarize current studies surrounding this controversy on necroptosis-based antitumor research and discuss the advantages, potential issues, and countermeasures of this novel therapy. VSports手机版.
"VSports在线直播" Figures
References
-
- Chan FK-M. Programmed necrosis/necroptosis: an inflammatory form of cell death. Cell Death. Springer, 2014: 211–228.
-
- Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 2014; 15: 135–147. - PubMed
-
- Jouan-Lanhouet S, Riquet F, Duprez L, Berghe TV, Takahashi N, Vandenabeele P. Necroptosis, in vivo detection in experimental disease models. Semin Cell Dev Biol 2014; 35: 2–13. - V体育官网入口 - PubMed
Publication types
- Actions (VSports注册入口)
MeSH terms
- "V体育官网" Actions
- "V体育安卓版" Actions
- "V体育平台登录" Actions
- "VSports在线直播" Actions
Substances (VSports在线直播)
- "VSports手机版" Actions
LinkOut - more resources
Full Text Sources
"V体育官网" Other Literature Sources
Miscellaneous

