Inclusion of Strep-tag II in design of antigen receptors for T-cell immunotherapy
- PMID: 26900664
- PMCID: PMC4940167
- DOI: 10.1038/nbt.3461 (V体育官网入口)
Inclusion of Strep-tag II in design of antigen receptors for T-cell immunotherapy
Abstract
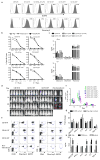
Adoptive immunotherapy with genetically engineered T cells has the potential to treat cancer and other diseases. The introduction of Strep-tag II sequences into specific sites in synthetic chimeric antigen receptors or natural T-cell receptors of diverse specificities provides engineered T cells with a marker for identification and rapid purification, a method for tailoring spacer length of chimeric receptors for optimal function, and a functional element for selective antibody-coated, microbead-driven, large-scale expansion. These receptor designs facilitate cGMP manufacturing of pure populations of engineered T cells for adoptive T-cell therapies and enable in vivo tracking and retrieval of transferred cells for downstream research applications. VSports手机版.
Conflict of interest statement (VSports注册入口)
L. L. and S. R V体育安卓版. R. are co-inventors of a patent “Tagged chimeric effector molecules and receptors thereof” filed by Fred Hutchinson Cancer Research Center (PCT/US2014/072007), and licensed to Juno Therapeutics.
S. R. R V体育ios版. holds equity stake in, and is a cofounder of, Juno Therapeutics and is on the advisory board for and consults for Cell Medica.
No potential conflicts of interest were disclosed by the other authors.
Figures


References
-
- Kochenderfer JN, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:540–549. doi: 10.1200/jco.2014.56.2025. - DOI - PMC - PubMed
-
- Robbins PF, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:1019–1027. doi: 10.1158/1078-0432.ccr-14-2708. - DOI - PMC - PubMed
Publication types
- "VSports在线直播" Actions
MeSH terms
- Actions (VSports注册入口)
- Actions (V体育2025版)
- "VSports app下载" Actions
- "V体育官网入口" Actions
- "V体育2025版" Actions
- "V体育ios版" Actions
Substances
- VSports手机版 - Actions
- Actions (VSports)
- "VSports在线直播" Actions
Grants and funding
LinkOut - more resources
"V体育安卓版" Full Text Sources
Other Literature Sources

