Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients (VSports注册入口)
- PMID: 26837580
- PMCID: PMC4738248
- DOI: 10.1038/srep20358
Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients
Abstract
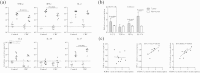
Mucosal associated invariant T (MAIT) cells are important for immune defense against infectious pathogens and regulate the pathogenesis of various inflammatory diseases. However, their roles in the development of colorectal cancer (CRC) are still unclear. This study examined the phenotype, distribution, clinical relevance and potential function of MAIT cells in CRC patients. We found that the percentages of circulating memory CD8(+) MAIT cells were significantly reduced while tumor infiltrating MAIT cells were increased, especially in patients with advanced CRC. The serum CEA levels were positively correlated with the percentages of tumor infiltrating MAIT cells in CRC patients, but negatively correlated with the percentages of circulating MAIT in advanced CRC patients. Activated circulating MAIT cells from CRC patients produced lower IFN-γ, but higher IL-17. Furthermore, higher levels of Vα7. 2-Jα33, IFN-γ and IL-17A were expressed in the CRC tissues. Co-culture of activated MAIT cells with HCT116 cells enhanced IL-17 expression and induced HCT116 cell cycle arrest at G2/M phase in a contact- and dose-dependent manner, which was abrogated by treatment with anti-MR1 VSports手机版. Therefore, MAIT cells preferably infiltrate into the solid tumor in CRC patients and may participate in the immune surveillance of CRC. .
"V体育官网入口" Figures




References
-
- Treiner E. et al.. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 422, 164–169 (2003). - PubMed
-
- Le Bourhis L. et al.. Antimicrobial activity of mucosal-associated invariant T cells. Nature immunology. 11, 701–708 (2010). - PubMed (VSports最新版本)
-
- Tang X. Z. et al.. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. Journal of immunology. 190, 3142–3152 (2013). - PubMed
-
- Le Bourhis L. et al.. Mucosal-associated invariant T cells: unconventional development and function. Trends in immunology. 32, 212–218 (2011). - PubMed
MeSH terms
- "VSports最新版本" Actions
- "V体育安卓版" Actions
- "V体育2025版" Actions
- Actions (V体育平台登录)
- VSports最新版本 - Actions
- Actions (V体育ios版)
"VSports在线直播" Substances
LinkOut - more resources
"V体育官网" Full Text Sources
V体育官网 - Other Literature Sources
"V体育官网" Medical
Molecular Biology Databases (VSports在线直播)
Research Materials

