VSports app下载 - Soluble Tumor Necrosis Factor Receptor 1 Released by Skin-Derived Mesenchymal Stem Cells Is Critical for Inhibiting Th17 Cell Differentiation
- PMID: 26819253
- PMCID: "VSports app下载" PMC4807667
- DOI: 10.5966/sctm.2015-0179
Soluble Tumor Necrosis Factor Receptor 1 Released by Skin-Derived Mesenchymal Stem Cells Is Critical for Inhibiting Th17 Cell Differentiation
Abstract
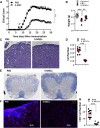
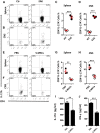
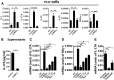
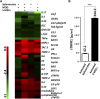
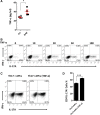
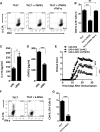
T helper 17 (Th17) cells play an important role in multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE). Th17 cell differentiation from naïve T cells can be induced in vitro by the cytokines transforming growth factor β1 and interleukin-6. However, it remains unclear whether other regulatory factors control the differentiation of Th17 cells. Mesenchymal stem cells (MSCs) have emerged as a promising candidate for inhibiting Th17 cell differentiation and autoimmune diseases. Despite the fact that several molecules have been linked to the immunomodulatory function of MSCs, many other key MSC-secreted regulators that are involved in inhibiting Th17 cell polarization are ill-defined. In this study, we demonstrated that the intraperitoneal administration of skin-derived MSCs (S-MSCs) substantially ameliorated the development of EAE in mice. We found that the proinflammatory cytokine tumor necrosis factor (TNF)-α, a key mediator in the pathophysiology of MS and EAE, was capable of promoting Th17 cell differentiation VSports手机版. Moreover, under inflammatory conditions, we demonstrated that S-MSCs produced high amounts of soluble TNF receptor 1 (sTNFR1), which binds TNF-α and antagonizes its function. Knockdown of sTNFR1 in S-MSCs decreased their inhibitory effect on Th17 cell differentiation ex vivo and in vivo. Thus, our data identified sTNFR1 and its target TNF-α as critical regulators for Th17 cell differentiation, suggesting a previously unrecognized mechanism for MSC therapy in Th17-mediated autoimmune diseases. .
Keywords: Autoimmune disease; Differentiation; T cells; Tissue-specific stem cells V体育安卓版. .
©AlphaMed Press.
Figures
References
-
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. - PubMed
-
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. - "VSports" PubMed
-
- Ke F, Zhang L, Liu Z, et al. Autocrine interleukin-6 drives skin-derived mesenchymal stem cell trafficking via regulating voltage-gated Ca(2+) channels. Stem Cells. 2014;32:2799–2810. - PubMed
-
- Sioud M, Mobergslien A, Boudabous A, et al. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol. 2010;71:267–274. - PubMed
-
- Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. - PubMed
Publication types
- Actions (V体育平台登录)
MeSH terms
- "V体育2025版" Actions
- Actions (VSports app下载)
- VSports注册入口 - Actions
- "VSports最新版本" Actions
- V体育安卓版 - Actions
- "VSports最新版本" Actions
- "V体育ios版" Actions
- VSports app下载 - Actions
- "VSports" Actions
- "VSports注册入口" Actions
- "V体育平台登录" Actions
- Actions (V体育ios版)
- "VSports" Actions
Substances
- Actions (V体育2025版)
- "V体育安卓版" Actions
- "VSports手机版" Actions
LinkOut - more resources
Full Text Sources (V体育安卓版)
Other Literature Sources
Medical (VSports最新版本)

