Recruitment of A20 by the C-terminal domain of NEMO suppresses NF-κB activation and autoinflammatory disease
- PMID: 26802121
- PMCID: PMC4760784
- DOI: 10.1073/pnas.1518163113
"VSports手机版" Recruitment of A20 by the C-terminal domain of NEMO suppresses NF-κB activation and autoinflammatory disease
Abstract
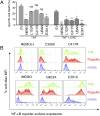
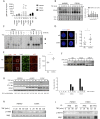
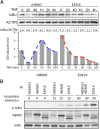
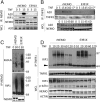
Receptor-induced NF-κB activation is controlled by NEMO, the NF-κB essential modulator. Hypomorphic NEMO mutations result in X-linked ectodermal dysplasia with anhidrosis and immunodeficiency, also referred to as NEMO syndrome. Here we describe a distinct group of patients with NEMO C-terminal deletion (ΔCT-NEMO) mutations. Individuals harboring these mutations develop inflammatory skin and intestinal disease in addition to ectodermal dysplasia with anhidrosis and immunodeficiency. Both primary cells from these patients, as well as reconstituted cell lines with this deletion, exhibited increased IκB kinase (IKK) activity and production of proinflammatory cytokines. Unlike previously described loss-of-function mutations, ΔCT-NEMO mutants promoted increased NF-κB activation in response to TNF and Toll-like receptor stimulation. Investigation of the underlying mechanisms revealed impaired interactions with A20, a negative regulator of NF-κB activation, leading to prolonged accumulation of K63-ubiquitinated RIP within the TNFR1 signaling complex. Recruitment of A20 to the C-terminal domain of NEMO represents a novel mechanism limiting NF-κB activation by NEMO, and its absence results in autoinflammatory disease VSports手机版. .
Keywords: A20; HED-ID; NF-κB; TLR; autoinflammatory disease. V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
"V体育官网" Figures




Comment in
-
Inflammation: A20-NEMO interaction prevents autoinflammation.Nat Rev Rheumatol. 2016 Mar;12(3):134. doi: 10.1038/nrrheum.2016.19. Epub 2016 Feb 11. Nat Rev Rheumatol. 2016. PMID: 26865109 No abstract available.
VSports最新版本 - References
-
- Ruland J. Return to homeostasis: Downregulation of NF-κB responses. Nat Immunol. 2011;12(8):709–714. - VSports - PubMed
-
- Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8(4):398–406. - PubMed
MeSH terms
- "VSports注册入口" Actions
- Actions (VSports)
- "VSports手机版" Actions
- "VSports在线直播" Actions
- VSports app下载 - Actions
- "V体育2025版" Actions
- VSports - Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- VSports注册入口 - Actions
- V体育安卓版 - Actions
- "V体育官网" Actions
- Actions (V体育官网)
- Actions (V体育2025版)
- "VSports手机版" Actions
Substances
- V体育安卓版 - Actions
- "V体育安卓版" Actions
- Actions (VSports)
- "VSports app下载" Actions
- "V体育ios版" Actions
- Actions (VSports在线直播)
- Actions (V体育ios版)
- "V体育官网" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases (V体育2025版)
Miscellaneous

