A Highly Conserved Bacterial D-Serine Uptake System Links Host Metabolism and Virulence (V体育官网入口)
- PMID: 26727373
- PMCID: PMC4699771
- DOI: VSports在线直播 - 10.1371/journal.ppat.1005359
"VSports手机版" A Highly Conserved Bacterial D-Serine Uptake System Links Host Metabolism and Virulence
"V体育官网入口" Abstract
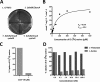
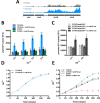
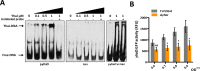
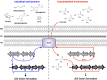
The ability of any organism to sense and respond to challenges presented in the environment is critically important for promoting or restricting colonization of specific sites. Recent work has demonstrated that the host metabolite D-serine has the ability to markedly influence the outcome of infection by repressing the type III secretion system of enterohaemorrhagic Escherichia coli (EHEC) in a concentration-dependent manner VSports手机版. However, exactly how EHEC monitors environmental D-serine is not understood. In this work, we have identified two highly conserved members of the E. coli core genome, encoding an inner membrane transporter and a transcriptional regulator, which collectively help to "sense" levels of D-serine by regulating its uptake from the environment and in turn influencing global gene expression. Both proteins are required for full expression of the type III secretion system and diversely regulated prophage-encoded effector proteins demonstrating an important infection-relevant adaptation of the core genome. We propose that this system acts as a key safety net, sampling the environment for this metabolite, thereby promoting colonization of EHEC to favorable sites within the host. .
Conflict of interest statement (VSports手机版)
The authors have declared that no competing interests exist.
VSports - Figures

Comment in
-
When and where? Pathogenic Escherichia coli differentially sense host D-serine using a universal transporter system to monitor their environment.Microb Cell. 2016 Mar 31;3(4):181-184. doi: 10.15698/mic2016.04.494. Microb Cell. 2016. PMID: 28357351 Free PMC article.
References
-
- Kaper JB, Nataro JP, Mobley HL (2004) Pathogenic Escherichia coli. Nat Rev Microbiol 2: 123–140. 10.1038/nrmicro818 - "V体育官网" DOI - PubMed
-
- Van Elsas JD, Semenov A V, Costa R, Trevors JT (2011) Survival of Escherichia coli in the environment: fundamental and public health aspects. ISME J 5: 173–183. 10.1038/ismej.2010.80 - DOI (V体育官网入口) - PMC - PubMed
-
- Croxen MA, Finlay BB (2010) Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol 8: 26–38. 10.1038/nrmicro2265 - DOI (V体育平台登录) - PubMed
Publication types
MeSH terms
- "VSports手机版" Actions
- V体育安卓版 - Actions
- V体育ios版 - Actions
- "VSports最新版本" Actions
- Actions (VSports在线直播)
- VSports app下载 - Actions
- "V体育官网入口" Actions
- "VSports注册入口" Actions
- V体育ios版 - Actions
- VSports最新版本 - Actions
Substances
- "VSports注册入口" Actions
- Actions (V体育ios版)
Grants and funding (VSports app下载)
LinkOut - more resources (VSports)
Full Text Sources
Other Literature Sources
"VSports注册入口" Medical
Molecular Biology Databases

