Perturbing the Copper(III)-Hydroxide Unit through Ligand Structural Variation (VSports手机版)
- PMID: 26693733
- PMCID: PMC4857600
- DOI: 10.1021/jacs.5b10985
V体育2025版 - Perturbing the Copper(III)-Hydroxide Unit through Ligand Structural Variation
Abstract
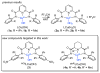
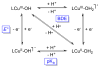
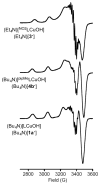
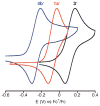
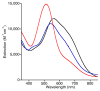
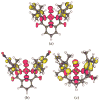
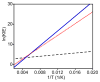
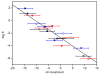
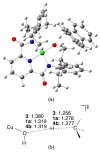
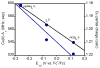
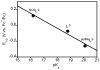
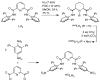
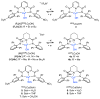
Two new ligand sets, (pipMe)LH2 and (NO2)LH2 ((pipMe)L = N,N'-bis(2,6-diisopropylphenyl)-1-methylpiperidine-2,6-dicarboxamide, (NO2)L = N,N'-bis(2,6-diisopropyl-4-nitrophenyl)pyridine-2,6-dicarboxamide), are reported which are designed to perturb the overall electronics of the copper(III)-hydroxide core and the resulting effects on the thermodynamics and kinetics of its hydrogen-atom abstraction (HAT) reactions VSports手机版. Bond dissociation energies (BDEs) for the O-H bonds of the corresponding Cu(II)-OH2 complexes were measured that reveal that changes in the redox potential for the Cu(III)/Cu(II) couple are only partially offset by opposite changes in the pKa, leading to modest differences in BDE among the three compounds. The effects of these changes were further probed by evaluating the rates of HAT by the corresponding Cu(III)-hydroxide complexes from substrates with C-H bonds of variable strength. These studies revealed an overarching linear trend in the relationship between the log k (where k is the second-order rate constant) and the ΔH of reaction. Additional subtleties in measured rates arise, however, that are associated with variations in hydrogen-atom abstraction barrier heights and tunneling efficiencies over the temperature range from -80 to -20 °C, as inferred from measured kinetic isotope effects and corresponding electronic-structure-based transition-state theory calculations. .
Conflict of interest statement
The authors declare no competing financial interest.
Figures
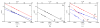
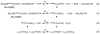
References
-
- Solomon EI, Heppner DE, Johnston EM, Ginsbach JW, Cirera J, Qayyum M, Kieber-Emmons MT, Kjaergaard CH, Hadt RG, Tian L. Chem Rev. 2014;114:3659–3853. - PMC - PubMed
- Solomon E, Sarangi R, Woertink J, Augustine A, Yoon J, Ghosh S. Acc Chem Res. 2007;40:581–591. - PMC - PubMed
- Solomon EI, Sundaram UM, Machonkin TE. Chem Rev. 1996;96:2563–2605. - PubMed (VSports手机版)
- Klinman JP. Chem Rev. 1996;96:2541–2561. - PubMed
-
- Wendlandt AE, Suess AM, Stahl SS. Angew Chem, Int Ed. 2011;50:11062–11087. - PubMed
- Alayon EMC, Nachtegaal M, Ranocchiari M, van Bokhoven JA. Chimia. 2012;66:668–674. - "V体育平台登录" PubMed
- Vanelderen P, Hadt RG, Smeets PJ, Solomon EI, Schoonheydt RA, Sels BF. J Catal. 2011;284:157–164. - PMC (VSports手机版) - PubMed
-
- Mirica LM, Ottenwaelder X, Stack TDP. Chem Rev. 2004;104:1013–1045. - PubMed
- Lewis EA, Tolman WB. Chem Rev. 2004;104:1047–1076. - PubMed
- Itoh S. Curr Opin Chem Biol. 2006;10:115–122. - PubMed
- Cramer CJ, Tolman WB. Acc Chem Res. 2007;40:601–608. - PMC - PubMed
- Himes RA, Karlin KD. Curr Opin Chem Biol. 2009;13:119–131. - PMC - PubMed
-
-
Illustrative recent examples: Kim S, Saracini C, Siegler MA, Drichko N, Karlin KD. Inorg Chem. 2012;51:12603–12605 V体育平台登录. Kunishita A, Ertem MZ, Okubo Y, Tano T, Sugimoto H, Ohkubo K, Fujieda N, Fukuzumi S, Cramer CJ, Itoh S. Inorg Chem. 2012;51:9465–9480.
-
-
- Decker A, Solomon EI. Curr Opin Chem Biol. 2005;9:152–163. - PubMed
- Schroder D, Holthausen MC, Schwarz H. J Phys Chem B. 2004;108:14407–14416.
- Dietl N, Schlangen M, Schwarz H. Angew Chem, Int Ed. 2012;51:5544–5555. - VSports在线直播 - PubMed
- Kamachi T, Kihara N, Shiota Y, Yoshizawa K. Inorg Chem. 2005;44:4226–4236. - PubMed
- Yoshizawa K, Kihara N, Kamachi T, Shiota Y. Inorg Chem. 2006;45:3034–3041. - PubMed (VSports app下载)
- Comba P, Knoppe S, Martin B, Rajaraman G, Rolli C, Shapiro B, Stork T. Chem - Eur J. 2008;14:344–357. - PubMed
- Kim S, Ståhlberg J, Sandgren M. Proc Natl Acad Sci U S A. 2014;111:149–154. - PMC - PubMed
Publication types
- V体育官网 - Actions
- V体育安卓版 - Actions
MeSH terms
- VSports最新版本 - Actions
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports注册入口" Miscellaneous

