V体育安卓版 - pH-Dependent Equilibrium between 5-Guanidinohydantoin and Iminoallantoin Affects Nucleotide Insertion Opposite the DNA Lesion
- PMID: 26582419
- PMCID: PMC4802493
- DOI: 10.1021/acs.joc.5b02180
"V体育安卓版" pH-Dependent Equilibrium between 5-Guanidinohydantoin and Iminoallantoin Affects Nucleotide Insertion Opposite the DNA Lesion
Abstract
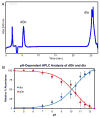
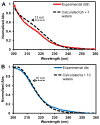
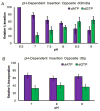
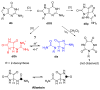
Four-electron oxidation of 2'-deoxyguanosine (dG) yields 5-guanidinohydantoin (dGh) as a product. Previously, we hypothesized that dGh could isomerize to iminoallantoin (dIa) via a mechanism similar to the isomerization of allantoin. The isomerization reaction was monitored by HPLC and found to be pH dependent with a transition pH = 10. 1 in which dGh was favored at low pH and dIa was favored at high pH. The structures for these isomers were confirmed by UV-vis, MS, and (1)H and (13)C NMR. Additionally, the UV-vis and NMR experimental results are supported by density functional theory calculations. A mechanism is proposed to support the pH dependency of the isomerization reaction. Next, we noted the hydantoin ring of dGh mimics thymine, while the iminohydantoin ring of dIa mimics cytosine; consequently, a dGh/dIa site was synthesized in a DNA template strand, and standing start primer extension studies were conducted with Klenow fragment exo(-). The dATP/dGTP insertion ratio opposite the dGh/dIa site as a function of pH was evaluated from pH 6. 5-9. 0. At pH 6 VSports手机版. 5, only dATP was inserted, but as the pH increased to 9. 0, the amount of dGTP insertion steadily increased. This observation supports dGh to dIa isomerization in DNA with a transition pH of ∼8. 2. .
Conflict of interest statement
The authors declare no competing financial interests.
Figures

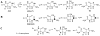
"VSports注册入口" References
-
- Cadet J, Wagner JR, Shafirovich V, Geacintov NE. Int J Radiat Biol. 2014;90:423–432. - "V体育2025版" PMC - PubMed
-
- Gates K. Chem Res Toxicol. 2009;22:1747–1760. - PMC (VSports) - PubMed
-
- Kunkel TA. Curr Opin Chem Biol. 2011;15:620–626. - "V体育平台登录" PMC - PubMed
-
- Saxowsky TT, Doetsch PW. Chem Rev. 2006;106:474–488. - "VSports注册入口" PubMed
Publication types
- "VSports手机版" Actions
- Actions (V体育平台登录)
MeSH terms (VSports注册入口)
- "VSports app下载" Actions
- "VSports最新版本" Actions
- VSports注册入口 - Actions
- Actions (VSports在线直播)
Substances
- Actions (V体育ios版)
- "V体育安卓版" Actions
"V体育ios版" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

