The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps (V体育2025版)
- PMID: 26492565
- PMCID: "VSports" PMC4619649
- DOI: 10.1371/journal.ppat.1005187 (V体育2025版)
The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps
Abstract
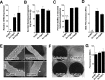
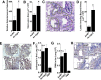
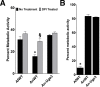
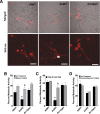
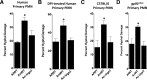
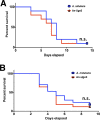
Of the over 250 Aspergillus species, Aspergillus fumigatus accounts for up to 80% of invasive human infections. A. fumigatus produces galactosaminogalactan (GAG), an exopolysaccharide composed of galactose and N-acetyl-galactosamine (GalNAc) that mediates adherence and is required for full virulence. Less pathogenic Aspergillus species were found to produce GAG with a lower GalNAc content than A. fumigatus and expressed minimal amounts of cell wall-bound GAG. Increasing the GalNAc content of GAG of the minimally pathogenic A. nidulans, either through overexpression of the A. nidulans epimerase UgeB or by heterologous expression of the A. fumigatus epimerase Uge3 increased the amount of cell wall bound GAG, augmented adherence in vitro and enhanced virulence in corticosteroid-treated mice to levels similar to A. fumigatus. The enhanced virulence of the overexpression strain of A. nidulans was associated with increased resistance to NADPH oxidase-dependent neutrophil extracellular traps (NETs) in vitro, and was not observed in neutropenic mice or mice deficient in NADPH-oxidase that are unable to form NETs. Collectively, these data suggest that cell wall-bound GAG enhances virulence through mediating resistance to NETs VSports手机版. .
Conflict of interest statement
The authors have declared that no competing interests exist.
V体育官网入口 - Figures



References
-
- Garcia-Vidal C, Upton A, Kirby KA, Marr KA (2008) Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 47: 1041–1050. - PMC - PubMed
-
- Lucas GM, Tucker P, Merz WG (1999) Primary Cutaneous Aspergillus nidulans Infection Associated with a Hickman Catheter in a Patient with Neutropenia. Clinical Infectious Diseases 29: 1594–1596. - PubMed
-
- Henriet SS, Verweij PE, Warris A (2012) Aspergillus nidulans and chronic granulomatous disease: a unique host-pathogen interaction. J Infect Dis 206: 1128–1137. 10.1093/infdis/jis473 - V体育安卓版 - DOI - PubMed
Publication types
- Actions (V体育官网入口)
- "VSports注册入口" Actions
MeSH terms
- "VSports" Actions
- "V体育2025版" Actions
- VSports注册入口 - Actions
- "VSports在线直播" Actions
- "V体育官网入口" Actions
- VSports在线直播 - Actions
- "VSports app下载" Actions
Substances
- V体育平台登录 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

