IL-4 Induced Innate CD8+ T Cells Control Persistent Viral Infection
- PMID: 26452143
- PMCID: "VSports" PMC4599894
- DOI: 10.1371/journal.ppat.1005193
IL-4 Induced Innate CD8+ T Cells Control Persistent Viral Infection
Abstract
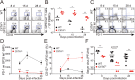
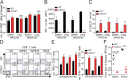
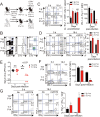
Memory-like CD8+ T cells expressing eomesodermin are a subset of innate T cells initially identified in a number of genetically modified mice, and also exist in wild mice and human. The acquisition of memory phenotype and function by these T cells is dependent on IL-4 produced by PLZF+ innate T cells; however, their physiologic function is still not known. Here we found that these IL-4-induced innate CD8+ T cells are critical for accelerating the control of chronic virus infection. In CIITA-transgenic mice, which have a substantial population of IL-4-induced innate CD8+ T cells, this population facilitated rapid control of viremia and induction of functional anti-viral T-cell responses during infection with chronic form of lymphocytic choriomeningitis virus. Characteristically, anti-viral innate CD8+ T cells accumulated sufficiently during early phase of infection. They produced a robust amount of IFN-γ and TNF-α with enhanced expression of a degranulation marker. Furthermore, this finding was confirmed in wild-type mice. Taken together, the results from our study show that innate CD8+ T cells works as an early defense mechanism against chronic viral infection VSports手机版. .
"V体育官网" Conflict of interest statement
The authors have declared that no competing interests exist.
VSports在线直播 - Figures





V体育2025版 - References
-
- Berg LJ (2007) Signalling through TEC kinases regulates conventional versus innate CD8+ T-cell development. Nat Rev Immunol 7: 479–485. - PubMed
-
- Veillette A, Dong Z, Latour S (2007) Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity 27: 698–710. - PubMed
-
- Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ (2006) The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity 25: 79–91. - "VSports app下载" PubMed
-
- Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, et al. (2006) Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity 25: 93–104. - PubMed (V体育2025版)
Publication types
- Actions (VSports手机版)
MeSH terms
- Actions (V体育2025版)
- VSports app下载 - Actions
- V体育官网入口 - Actions
- "VSports最新版本" Actions
- Actions (VSports注册入口)
- VSports在线直播 - Actions
- V体育官网入口 - Actions
- VSports app下载 - Actions
- Actions (V体育平台登录)
- Actions (VSports注册入口)
"VSports最新版本" Substances
- "VSports app下载" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

