Generation of Th17 cells in response to intranasal infection requires TGF-β1 from dendritic cells and IL-6 from CD301b+ dendritic cells
- PMID: 26417101
- PMCID: PMC4611596
- DOI: 10.1073/pnas.1513532112
Generation of Th17 cells in response to intranasal infection requires TGF-β1 from dendritic cells and IL-6 from CD301b+ dendritic cells
Abstract
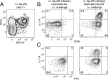
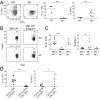
Intranasal (i. n. ) infections preferentially generate Th17 cells VSports手机版. We explored the basis for this anatomic preference by tracking polyclonal CD4(+) T cells specific for an MHC class II-bound peptide from the mucosal pathogen Streptococcus pyogenes. S. pyogenes MHC class II-bound peptide-specific CD4(+) T cells were first activated in the cervical lymph nodes following i. n. inoculation and then differentiated into Th17 cells. S. pyogenes-induced Th17 formation depended on TGF-β1 from dendritic cells and IL-6 from a CD301b(+) dendritic cell subset located in the cervical lymph nodes but not the spleen. Thus, the tendency of i. n. infection to induce Th17 cells is related to cytokine production by specialized dendritic cells that drain this site. .
Keywords: CD301b+; Streptococcus pyogenes; Th17; dendritic cells V体育安卓版. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. - PubMed
-
- Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. - PubMed
-
- Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. - PubMed
-
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. - PubMed
-
- Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26(5):579–591. - "VSports最新版本" PubMed
Publication types
V体育安卓版 - MeSH terms
- Actions (V体育2025版)
- VSports最新版本 - Actions
- Actions (V体育2025版)
- VSports - Actions
- VSports手机版 - Actions
- V体育官网 - Actions
Substances
- Actions (V体育官网)
- Actions (V体育平台登录)
- "VSports最新版本" Actions
- V体育官网 - Actions
"VSports在线直播" Grants and funding
"VSports" LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials (V体育平台登录)

