Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death
- PMID: 26405158
- PMCID: PMC4695193
- DOI: 10.18632/oncotarget.5162
Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death (VSports手机版)
VSports在线直播 - Abstract
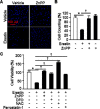
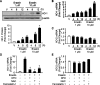
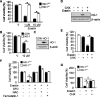
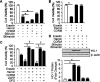
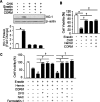
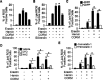
The oncogenic RAS-selective lethal small molecule Erastin triggers a unique iron-dependent form of nonapoptotic cell death termed ferroptosis. Ferroptosis is dependent upon the production of intracellular iron-dependent reactive oxygen species (ROS), but not other metals. However, key regulators remain unknown. The heme oxygenase (HO) is a major intracellular source of iron. In this study, the role of heme oxygenase in Erastin-triggered ferroptotic cancer cell death has been investigated VSports手机版. Zinc protoporphyrin IX (ZnPP), a HO-1 inhibitor, prevented Erastin-triggered ferroptotic cancer cell death. Furthermore, Erastin induced the protein and mRNA levels of HO-1 in HT-1080 fibrosarcoma cells. HO-1+/+ and HO-1-/- fibroblast, HO-1 overexpression, and chycloheximide-treated experiments revealed that the expression of HO-1 has a decisive effects in Erastin-triggered cell death. Hemin and CO-releasing molecules (CORM) promote Erastin-induced ferroptotic cell death, not by biliverdin and bilirubin. In addition, hemin and CORM accelerate the HO-1 expression in the presence of Erastin and increase membranous lipid peroxidation. Thus, HO-1 is an essential enzyme for iron-dependent lipid peroxidation during ferroptotic cell death. .
Keywords: free radicals; heme oxygenase-1; iron; oncogene; oncology V体育安卓版. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. - PubMed
-
- Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. - PubMed
-
- Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–245. - "V体育2025版" PMC - PubMed
Publication types
MeSH terms
- V体育官网入口 - Actions
- "V体育平台登录" Actions
- VSports最新版本 - Actions
- Actions (V体育官网入口)
- V体育ios版 - Actions
- "VSports手机版" Actions
- Actions (V体育ios版)
- "V体育官网" Actions
- "VSports app下载" Actions
- "VSports手机版" Actions
Substances (V体育官网入口)
- Actions (V体育2025版)
- "V体育ios版" Actions
- Actions (VSports注册入口)
- VSports app下载 - Actions
- "VSports app下载" Actions
- V体育安卓版 - Actions
- "VSports在线直播" Actions
- "VSports app下载" Actions
- "V体育平台登录" Actions
- Actions (V体育2025版)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

