SOD1 misplacing and mitochondrial dysfunction in amyotrophic lateral sclerosis pathogenesis
- PMID: 26379505
- PMCID: "VSports在线直播" PMC4548205
- DOI: "V体育安卓版" 10.3389/fncel.2015.00336
SOD1 misplacing and mitochondrial dysfunction in amyotrophic lateral sclerosis pathogenesis
Abstract
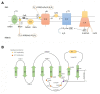
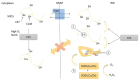
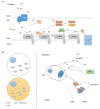
Amyotrophic lateral sclerosis (ALS) is a fatal motor neuron disease presenting as sporadic (sALS) or familial (fALS) forms. Even if the list of the genes underlining ALS greatly expanded, defects in superoxide dismutase 1 (SOD1), encoding the copper/zinc SOD1, still remain a major cause of fALS and are likely involved also in apparently sporadic presentations. The pathogenesis of ALS is still unknown, but several lines of evidence indicate that the mitochondrial accumulation of mutant SOD1 is an important mechanism of mitochondrial dysfunction, leading to motor neuron pathology and death. The intramitochondrial localization of mutant SOD1 is debated. Mutant SOD1 might accumulate inside the intermembrane space (IMS), overriding the physiological retention regulated by the copper chaperone for superoxide dismutase (CCS). On the other hand, misfolded SOD1 might deposit onto the outer mitochondrial membrane (OMM), clumping the transport across mitochondrial membranes and engaging mitochondrial-dependent cell apoptosis VSports手机版. The elucidation of the mechanisms ruling SOD1 localization and misplacing might shed light on peculiar ALS features such as cell selectivity and late onset. More importantly, these studies might disclose novel targets for therapeutic intervention in familial ALS as well as non-genetic forms. Finally, pharmacological or genetic manipulation aimed to prevent or counteract the intracellular shifting of mutant SOD1 could be effective for other neurodegenerative disorders featuring the toxic accumulation of misfolded proteins. .
Keywords: mitochondria; mitochondrial intermembrane space; motor neuron disorder; outer mitochondrial membrane; protein misfolding; superoxide dismutase 1 V体育安卓版. .
Figures
References (VSports)
-
- Bailey A. O., Miller T. M., Dong M. Q., Vande Velde C., Cleveland D. W., Yates J. R. (2007). RCADiA: simple automation platform for comparative multidimensional protein identification technology. Anal. Chem. 79, 6410–6418. 10.1021/ac070585g - VSports注册入口 - DOI - PMC - PubMed
Publication types
V体育平台登录 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

