ER stress induces NLRP3 inflammasome activation and hepatocyte death
- PMID: 26355342
- PMCID: "VSports" PMC4650444
- DOI: 10.1038/cddis.2015.248
"V体育官网入口" ER stress induces NLRP3 inflammasome activation and hepatocyte death
"VSports最新版本" Abstract
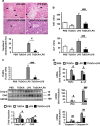
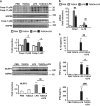
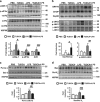
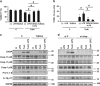
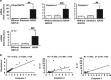
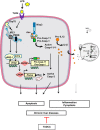
The incidence of chronic liver disease is constantly increasing, owing to the obesity epidemic. However, the causes and mechanisms of inflammation-mediated liver damage remain poorly understood. Endoplasmic reticulum (ER) stress is an initiator of cell death and inflammatory mechanisms. Although obesity induces ER stress, the interplay between hepatic ER stress, NLRP3 inflammasome activation and hepatocyte death signaling has not yet been explored during the etiology of chronic liver diseases. Steatosis is a common disorder affecting obese patients; moreover, 25% of these patients develop steatohepatitis with an inherent risk for progression to hepatocarcinoma. Increased plasma LPS levels have been detected in the serum of patients with steatohepatitis. We hypothesized that, as a consequence of increased plasma LPS, ER stress could be induced and lead to NLRP3 inflammasome activation and hepatocyte death associated with steatohepatitis progression. In livers from obese mice, administration of LPS or tunicamycin results in IRE1α and PERK activation, leading to the overexpression of CHOP VSports手机版. This, in turn, activates the NLRP3 inflammasome, subsequently initiating hepatocyte pyroptosis (caspase-1, -11, interleukin-1β secretion) and apoptosis (caspase-3, BH3-only proteins). In contrast, the LPS challenge is blocked by the ER stress inhibitor TUDCA, resulting in: CHOP downregulation, reduced caspase-1, caspase-11, caspase-3 activities, lowered interleukin-1β secretion and rescue from cell death. The central role of CHOP in mediating the activation of proinflammatory caspases and cell death was characterized by performing knockdown experiments in primary mouse hepatocytes. Finally, the analysis of human steatohepatitis liver biopsies showed a correlation between the upregulation of inflammasome and ER stress markers, as well as liver injury. We demonstrate here that ER stress leads to hepatic NLRP3 inflammasome pyroptotic death, thus contributing as a novel mechanism of inflammation-mediated liver injury in chronic liver diseases. Inhibition of ER-dependent inflammasome activation and cell death pathways may represent a potential therapeutic approach in chronic liver diseases. .
Figures

References (V体育平台登录)
-
- 2Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002; 346: 1221–1231. - PubMed
-
- 3Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306: 457–461. - PubMed
-
- 4Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 2008; 134: 568–576. - PubMed
-
- 5Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 2005; 115: 2656–2664. - "VSports注册入口" PMC - PubMed
"V体育平台登录" Publication types
- Actions (VSports注册入口)
MeSH terms
- VSports最新版本 - Actions
- Actions (V体育平台登录)
- V体育官网入口 - Actions
- Actions (V体育官网)
- Actions (VSports app下载)
Substances
- Actions (V体育平台登录)
- Actions (V体育安卓版)
- VSports在线直播 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

