Human calprotectin is an iron-sequestering host-defense protein
- PMID: 26302479
- PMCID: "V体育2025版" PMC4575267
- DOI: 10.1038/nchembio.1891 (VSports app下载)
Human calprotectin is an iron-sequestering host-defense protein
Abstract
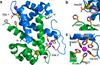
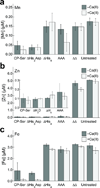
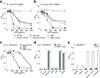
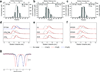
Human calprotectin (CP) is a metal-chelating antimicrobial protein of the innate immune response. The current working model states that CP sequesters manganese and zinc from pathogens. We report the discovery that CP chelates iron and deprives bacteria of this essential nutrient. Elemental analysis of CP-treated growth medium establishes that CP reduces the concentrations of manganese, iron and zinc. Microbial growth studies reveal that iron depletion by CP contributes to the growth inhibition of bacterial pathogens. Biochemical investigations demonstrate that CP coordinates Fe(II) at an unusual hexahistidine motif, and the Mössbauer spectrum of (57)Fe(II)-bound CP is consistent with coordination of high-spin Fe(II) at this site (δ = 1. 20 mm/s, ΔEQ = 1 VSports手机版. 78 mm/s). In the presence of Ca(II), CP turns on its iron-sequestering function and exhibits subpicomolar affinity for Fe(II). Our findings expand the biological coordination chemistry of iron and support a previously unappreciated role for CP in mammalian iron homeostasis. .
Figures


Comment in
-
Metals: Calprotectin and iron match up.Nat Chem Biol. 2015 Oct;11(10):756-7. doi: 10.1038/nchembio.1915. Nat Chem Biol. 2015. PMID: 26379021 No abstract available.
References
-
- Bertini I, Gray HB, Stiefel EI, Valentine JS. Biological inorganic chemistry: structure and reactivity. University Science Books; 2007.
-
- Weinberg ED. Nutritional immunity: host’s attempt to withhold iron from microbial invaders. J. Am. Med. Assoc. 1975;231:39–41. - PubMed
-
- Fischbach MA, Lin H, Liu DR, Walsh CT. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat. Chem. Biol. 2006;2:132–138. - PubMed
-
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012;10:525–537. - "V体育2025版" PMC - PubMed
Methods-only references
-
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine) Anal. Biochem. 1971;40:450–458. - PubMed
Publication types
- "V体育2025版" Actions
- Actions (V体育官网)
MeSH terms
- V体育安卓版 - Actions
- "VSports app下载" Actions
- "V体育官网入口" Actions
- V体育安卓版 - Actions
- Actions (V体育平台登录)
- Actions (VSports最新版本)
- "V体育ios版" Actions
- VSports app下载 - Actions
Substances
- Actions (VSports在线直播)
- "V体育官网入口" Actions
- "V体育官网" Actions
VSports手机版 - Grants and funding
LinkOut - more resources
VSports手机版 - Full Text Sources
Other Literature Sources
"VSports最新版本" Medical
Molecular Biology Databases
Miscellaneous

