The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines
- PMID: 26276366
- PMCID: "V体育安卓版" PMC4688306
- DOI: 10.1007/s10120-015-0526-8
The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines
Abstract
Although gastrointestinal stromal tumors (GISTs) are a rare type of cancer, they are the commonest sarcoma in the gastrointestinal tract. Molecularly targeted therapy, such as imatinib therapy, has revolutionized the treatment of advanced GIST and facilitates scientific research on GIST. Nevertheless, surgery remains a mainstay of treatment to obtain a permanent cure for GIST even in the era of targeted therapy. Many GIST guidelines have been published to guide the diagnosis and treatment of the disease. We review current versions of GIST guidelines published by the National Comprehensive Cancer Network, by the European Society for Medical Oncology, and in Japan. All clinical practice guidelines for GIST include recommendations based on evidence as well as on expert consensus. Most of the content is very similar, as represented by the following examples: GIST is a heterogeneous disease that may have mutations in KIT, PDGFRA, HRAS, NRAS, BRAF, NF1, or the succinate dehydrogenase complex, and these subsets of tumors have several distinctive features. Although there are some minor differences among the guidelines--for example, in the dose of imatinib recommended for exon 9-mutated GIST or the efficacy of antigen retrieval via immunohistochemistry--their common objectives regarding diagnosis and treatment are not only to improve the diagnosis of GIST and the prognosis of patients but also to control medical costs. This review describes the current standard diagnosis, treatment, and follow-up of GISTs based on the recommendations of several guidelines and expert consensus VSports手机版. .
Keywords: Consensus based; Evidence-based; Gastrointestinal stromal tumor; Guidelines. V体育安卓版.
Conflict of interest statement
Conflict of interest
T. Nishida has received funding for basic research from Novartis and Bayer, and honoraria for speeches from Bayer and Pfizer. J. -Y. Blay has received research support and honoraria from Novartis, Pfizer, and Bayer. Y. -K. Kang has received grants for research and honoraria for speeches from Novartis and Bayer V体育ios版. S. Hirota and Y. Kitagawa declare that they have no conflict of interest.
Ethical standards
All authors declare the trust and integrity of this review by their following the rules of good scientific practice, including the following: The manuscript has not been submitted to another journal simultaneously. The manuscript has not been published previously. A single study is not split into several parts to increase the quantity of submissions. No data have been fabricated or manipulated to support the conclusions. No data, text, or theories by others are presented as if they were those of the authors. Proper acknowledgments of other work have been given, quotation marks are used for verbatim copying of material, and permission has been secured for material that is copyrighted, if necessary. Consent to submit the article was received explicitly from all authors, as well as from the responsible authorities—tacitly or explicitly—at the institute/organization where the work was performed, before the work was submitted VSports最新版本. All authors contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the descriptions.
Figures
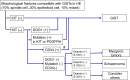
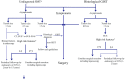
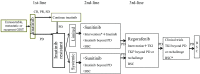
References (VSports手机版)
-
- Hirota S, Isozaki K, Moriyama Y, Kanakura Y, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Tunio GM, Matsuzawa Y, Shinomura Y, Kitamura Y. Gain-of-function mutation of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. - DOI - PubMed
-
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. - DOI - PubMed
-
- Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1–S41. - V体育安卓版 - PMC - PubMed
Publication types
- Actions (V体育安卓版)
MeSH terms
- Actions (V体育ios版)
- V体育官网入口 - Actions
- "VSports最新版本" Actions
- Actions (VSports最新版本)
- Actions (VSports app下载)
- Actions (V体育平台登录)
- Actions (VSports)
- VSports手机版 - Actions
V体育官网 - Substances
- Actions (VSports手机版)
- Actions (VSports)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

