The PI3K/AKT/mTOR pathway is a potential predictor of distinct invasive and migratory capacities in human ovarian cancer cell lines
- PMID: 26267321
- PMCID: V体育平台登录 - PMC4694849
- DOI: 10.18632/oncotarget.4550
The PI3K/AKT/mTOR pathway is a potential predictor of distinct invasive and migratory capacities in human ovarian cancer cell lines
Abstract
Objectives: To explore the genetic and molecular events that control subclones exhibiting distinct invasive/migratory capacities derived from human epithelial ovarian cancer (EOC) cell line A2780 and SKOV3. VSports手机版.
Methods: Single-cell subclones were isolated and established that were derived from the SKOV3 and A2780 cell lines through limiting dilution methodology. Transwell insert assays and MTT assays were performed to screen and identify the subclones exhibiting the highest and the lowest invasive/migratory capacities, and the selected subclones were renamed as A-H (A2780 high), A-L (A2780 low), S-H (SKOV3 high), and S-L (SKOV3 low). Their biological characteristics were evaluated. RNA-Seq was conducted on the targeted subclones. V体育安卓版.
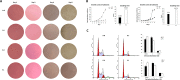
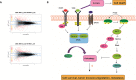
Results: Compared with their corresponding counterparts, A-H/S-H cells exhibited significantly higher invasive/migratory capacities (P < 0. 001 and = 0. 001, respectively). A-H/S-H cells displayed a clear reduction in doubling time (P = 0. 004 and 0. 001, respectively), and a significant increase in the percentage of cells in S phase (P = 0. 004 and 0. 022, respectively). Additionally, the apoptotic rates of A-H/S-H cells were significantly lower than those of A-L/S-L cells (P = 0. 002 and 0. 026, respectively). At both mRNA and protein levels, caspase-3 and caspase-7 expression were reduced but Bcl-2 expression was increased in A-H/S-H cells. The TrkB (anoikis-related) and Beclin1 (autophagy-related) levels were consistently high and low, respectively, in both A-H/S-H cells V体育ios版. Resistance to chemotherapy in vitro and higher capacities on tumor formation in vivo was presented in both A-H/S-H cells. PI3K/AKT/mTOR pathway components, PIK3CA, PIK3CD, AKT3, ECM1, GPCR, mTOR and PRKCB were increased but that the Nur77 and PTEN were decreased in A-H/S-H cells, identified by RNA-Seq and consistently confirmed by RT-PCR and Western blot analyses. .
Conclusions: Heterogeneous cell subpopulations exhibiting distinct invasive and migratory capacities co-exist within the SKOV3 and A2780 cell lines VSports最新版本. PI3K/AKT/mTOR pathway activation is associated with higher invasive and migratory capacities in subpopulations of human ovarian cancer cell lines. Inhibiting this pathway may be useful for the chemoprevention or treatment of EOC. .
Keywords: RNA-Seq; cell subclones; intratumoral heterogeneity; invasion and migration; ovarian cancer. V体育平台登录.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

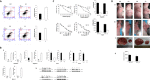

VSports注册入口 - References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer Journal international du cancer. 2015;136:E359–386. - PubMed
-
- McAlpine JN, Eisenkop SM, Spirtos NM. Tumor heterogeneity in ovarian cancer as demonstrated by in vitro chemoresistance assays. Gynecologic oncology. 2008;110:360–364. - PubMed
-
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366:883–892. - "VSports手机版" PMC - PubMed
-
- Nguyen HG, Ravid K. Tetraploidy/aneuploidy and stem cells in cancer promotion: The role of chromosome passenger proteins. Journal of cellular physiology. 2006;208:12–22. - "VSports app下载" PubMed
Publication types
- "V体育ios版" Actions
"V体育安卓版" MeSH terms
- Actions (V体育官网入口)
- "VSports app下载" Actions
- V体育安卓版 - Actions
- "V体育官网入口" Actions
- V体育官网入口 - Actions
- V体育官网入口 - Actions
- Actions (VSports注册入口)
- V体育ios版 - Actions
- Actions (V体育平台登录)
- VSports在线直播 - Actions
- V体育平台登录 - Actions
- "V体育平台登录" Actions
- Actions (V体育平台登录)
- V体育平台登录 - Actions
- VSports app下载 - Actions
- V体育官网 - Actions
- Actions (VSports app下载)
- VSports最新版本 - Actions
Substances
- "VSports最新版本" Actions
- Actions (VSports最新版本)
- Actions (V体育ios版)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
"V体育ios版" Research Materials
Miscellaneous

