Nutlin-3 sensitizes nasopharyngeal carcinoma cells to cisplatin-induced cytotoxicity
- PMID: 26252575
- PMCID: "VSports在线直播" PMC4564086
- DOI: 10.3892/or.2015.4177
Nutlin-3 sensitizes nasopharyngeal carcinoma cells to cisplatin-induced cytotoxicity
Abstract
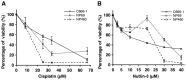
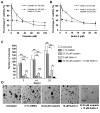
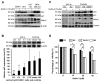
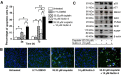
The small-molecule inhibitor of p53-Mdm2 interaction, Nutlin-3, is known to be effective against cancers expressing wild-type (wt) p53. p53 mutations are rare in nasopharyngeal carcinoma (NPC), hence targeting disruption of p53-Mdm2 interaction to reactivate p53 may offer a promising therapeutic strategy for NPC. In the present study, the effects of Nutlin-3 alone or in combination with cisplatin, a standard chemotherapeutic agent, were tested on C666-1 cells, an Epstein-Barr virus (EBV)-positive NPC cell line bearing wt p53. Treatment with Nutlin-3 activated the p53 pathway and sensitized NPC cells to the cytotoxic effects of cisplatin VSports手机版. The combined treatment also markedly suppressed soft agar colony growth formation and increased apoptosis of NPC cells. The effect of Nutlin-3 on NPC cells was inhibited by knockdown of p53, suggesting that its effect was p53-dependent. Extended treatment with increasing concentrations of Nutlin-3 did not result in emergence of p53 mutations in the C666-1 cells. Collectively, the present study revealed supportive evidence of the effectiveness of combining cisplatin and Nutlin-3 as a potential therapy against NPC. .
Figures
References
-
- Devi BC, Pisani P, Tang TS, Parkin DM. High incidence of nasopharyngeal carcinoma in native people of Sarawak, Borneo Island. Cancer Epidemiol Biomarkers Prev. 2004;13:482–486. - V体育官网入口 - PubMed
-
- Zeng MS, Zeng YX. Nasopharyngeal Cancer Medical Radiology. Springer Berlin; Heidelberg: 2010. Pathogenesis and etiology of nasopharyngeal carcinoma; pp. 9–25. - DOI
-
- Khoo AS, Pua KC. Nasopharyngeal Carcinoma: Keys for Translational Medicine and Biology. Landes Bioscience and Springer Science:Business Media; New York, NY: 2013. Diagnosis and clinical evaluation of nasopharyngeal carcinoma; pp. 1–9. - DOI
-
- Pua KC, Khoo AS, Yap YY, Subramaniam SK, Ong CA, Gopala Krishnan G, Shahid H, The Malaysian Nasopharyngeal Carcinoma Study Group Nasopharyngeal Carcinoma Database. Med J Malaysia. 2008;63(Suppl C):59–62. - "VSports app下载" PubMed
Publication types
- "V体育2025版" Actions
VSports手机版 - MeSH terms
- "V体育2025版" Actions
- "VSports手机版" Actions
- VSports最新版本 - Actions
- "V体育2025版" Actions
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- VSports - Actions
- "VSports app下载" Actions
Substances
- Actions (V体育ios版)
- VSports - Actions
- VSports最新版本 - Actions
- Actions (V体育官网入口)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials (V体育官网入口)
Miscellaneous

