Synthetic heme/copper assemblies: toward an understanding of cytochrome c oxidase interactions with dioxygen and nitrogen oxides
- PMID: 26244814
- PMCID: VSports注册入口 - PMC4779337
- DOI: 10.1021/acs.accounts.5b00265
Synthetic heme/copper assemblies: toward an understanding of cytochrome c oxidase interactions with dioxygen and nitrogen oxides
Abstract
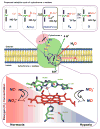
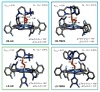
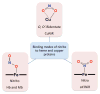
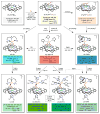
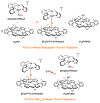
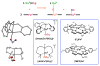
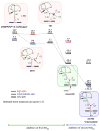
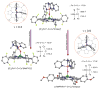
Our long-time niche in synthetic biological inorganic chemistry has been to design ligands and generate coordination complexes of copper or iron ions or both, those reacting with dioxygen (O2) or nitrogen oxides (e. g. , nitric oxide (NO(g)) and nitrite (NO2(-))) or both. As inspiration for this work, we turn to mitochondrial cytochrome c oxidase, which is responsible for dioxygen consumption and is also the predominant target for NO(g) and nitrite within mitochondria. In this Account, we highlight recent advances in studying synthetic heme/Cu complexes in two respects. First, there is the design, synthesis, and characterization of new O2 adducts whose further study will add insights into O2 reductive cleavage chemistry. Second, we describe how related heme/Cu constructs reduce nitrite ion to NO(g) or the reverse, oxidize NO(g) to nitrite. The reactions of nitrogen oxides occur as part of CcO's function, which is intimately tied to cellular O2 balance. We had first discovered that reduced heme/Cu compounds react with O2 giving μ-oxo heme-Fe(III)-O-Cu(II)(L) products; their properties are discussed. The O-atom is derived from dioxygen, and interrogations of these systems led to the construction and characterization of three distinctive classes of heme-peroxo complexes, two high-spin and one low-spin species. Recent investigations include a new approach to the synthesis of low-spin heme-peroxo-Cu complexes, employing a "naked" synthon, where the copper ligand denticity and geometric types can be varied. The result is a collection of such complexes; spectroscopic and structural features (by DFT calculations) are described. Some of these compounds are reactive toward reductants/protons effecting subsequent O-O cleavage. This points to how subtle improvements in ligand environment lead to a desired local structure and resulting optimized reactivity, as known to occur at enzyme active sites. The other sector of research is focused on heme/Cu assemblies mediating the redox interplay between nitrite and NO(g). In the nitrite reductase chemistry, the cupric center serves as a Lewis acid, while the heme is the redox active center providing the electron. The orientation of nitrite in approaching the ferrous heme center and N-atom binding are important. Also, detailed spectroscopic and kinetic studies of the NO(g) oxidase chemistry, in excellent agreement with theoretical calculations, reveal the intermediates and key mechanistic steps. Thus, we suggest that both chemical and biochemical heme/Cu-mediated nitrite reductase and NO(g) oxidase chemistry require N-atom binding to a ferrous heme along with cupric ion O-atom coordination, proceeding via a three-membered O-Fe-N chelate ring transition state. These important mechanistic features of heme/Cu systems interconverting NO(g) and nitrite are discussed for the first time. VSports手机版.
"VSports app下载" Figures
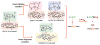

References
-
- Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Jei-Fei M, Libeu CP, Mizushima T, Yamaguchi H, Tomizaki T, Tsukihara T. Redox-Coupled Crystal Structure Changes in Bovine Heart Cytochrome c Oxidase. Science. 1998;280:1723–1729. - PubMed
-
- Kim E, Chufán EE, Kamaraj K, Karlin KD. Synthetic Models for Heme-Copper Oxidases. Chem Rev. 2004;104:1077–1133. - PubMed
-
- Yoshikawa S, Shimada A. Reaction Mechanism of Cytochrome c Oxidase. Chem Rev. 2015;115:1936–1989. - PubMed
-
- Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial Cytochrome Oxidase Produces Nitric Oxide under Hypoxic Conditions: Implications for Oxygen Sensing and Hypoxic Signaling in Eukaryotes. Cell Metab. 2006;3:277–287. - PubMed
Publication types
- "VSports注册入口" Actions
MeSH terms
- V体育2025版 - Actions
- Actions (VSports在线直播)
- Actions (VSports)
- "VSports手机版" Actions
VSports app下载 - Substances
- "VSports app下载" Actions
- V体育ios版 - Actions
- VSports最新版本 - Actions
Grants and funding
VSports最新版本 - LinkOut - more resources
Full Text Sources
"VSports在线直播" Other Literature Sources
"V体育ios版" Research Materials

