Active suppression of intestinal CD4(+)TCRαβ(+) T-lymphocyte maturation during the postnatal period (VSports注册入口)
- PMID: 26195040
- PMCID: PMC4518322 (VSports注册入口)
- DOI: 10.1038/ncomms8725
Active suppression of intestinal CD4(+)TCRαβ(+) T-lymphocyte maturation during the postnatal period
Abstract (VSports app下载)
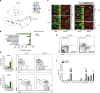
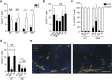
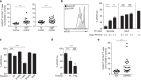
Priming of the mucosal immune system during the postnatal period substantially influences host-microbial interaction and susceptibility to immune-mediated diseases in adult life. The underlying mechanisms are ill defined. Here we show that shortly after birth, CD4 T cells populate preformed lymphoid structures in the small intestine and quickly acquire a distinct transcriptional profile. T-cell recruitment is independent of microbial colonization and innate or adaptive immune stimulation but requires β7 integrin expression. Surprisingly, neonatal CD4 T cells remain immature throughout the postnatal period under homeostatic conditions but undergo maturation and gain effector function on barrier disruption. Maternal SIgA and regulatory T cells act in concert to prevent immune stimulation and maintain the immature phenotype of CD4 T cells in the postnatal intestine during homeostasis. Active suppression of CD4 T-cell maturation during the postnatal period might contribute to prevent auto-reactivity, sustain a broad TCR repertoire and establish life-long immune homeostasis. VSports手机版.
Figures


References
-
- Garcia A. M., Fadel S. A., Cao S. & Sarzotti M. T cell immunity in neonates. Immunol. Res. 22, 177–190 (2000) . - PubMed
-
- Ishigame H. et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119 (2009) . - "V体育2025版" PubMed
-
- Palmer C., Bik E. M., DiGiulio D. B., Relman D. A. & Brown P. O. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177 (2007) . - PMC (V体育平台登录) - PubMed
V体育官网 - Publication types
- "V体育ios版" Actions
MeSH terms
- "VSports注册入口" Actions
- "V体育安卓版" Actions
- V体育平台登录 - Actions
LinkOut - more resources
"VSports app下载" Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

