"VSports app下载" Geometry of the Gene Expression Space of Individual Cells
- PMID: 26161936
- PMCID: PMC4498931
- DOI: 10.1371/journal.pcbi.1004224
Geometry of the Gene Expression Space of Individual Cells
Abstract
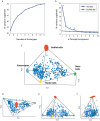
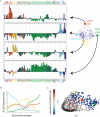
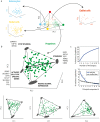
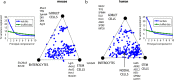
There is a revolution in the ability to analyze gene expression of single cells in a tissue. To understand this data we must comprehend how cells are distributed in a high-dimensional gene expression space. One open question is whether cell types form discrete clusters or whether gene expression forms a continuum of states. If such a continuum exists, what is its geometry. Recent theory on evolutionary trade-offs suggests that cells that need to perform multiple tasks are arranged in a polygon or polyhedron (line, triangle, tetrahedron and so on, generally called polytopes) in gene expression space, whose vertices are the expression profiles optimal for each task. Here, we analyze single-cell data from human and mouse tissues profiled using a variety of single-cell technologies VSports手机版. We fit the data to shapes with different numbers of vertices, compute their statistical significance, and infer their tasks. We find cases in which single cells fill out a continuum of expression states within a polyhedron. This occurs in intestinal progenitor cells, which fill out a tetrahedron in gene expression space. The four vertices of this tetrahedron are each enriched with genes for a specific task related to stemness and early differentiation. A polyhedral continuum of states is also found in spleen dendritic cells, known to perform multiple immune tasks: cells fill out a tetrahedron whose vertices correspond to key tasks related to maturation, pathogen sensing and communication with lymphocytes. A mixture of continuum-like distributions and discrete clusters is found in other cell types, including bone marrow and differentiated intestinal crypt cells. This approach can be used to understand the geometry and biological tasks of a wide range of single-cell datasets. The present results suggest that the concept of cell type may be expanded. In addition to discreet clusters in gene-expression space, we suggest a new possibility: a continuum of states within a polyhedron, in which the vertices represent specialists at key tasks. .
V体育官网入口 - Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Jaitin DA, Kenigsberg E, Keren-Shaul H, Elefant N, Paul F, Zaretsky I, et al. Massively Parallel Single-Cell RNA-Seq for Marker-Free Decomposition of Tissues into Cell Types. Science. 2014;343: 776–779. 10.1126/science.1247651 - DOI (V体育ios版) - PMC - PubMed
"V体育2025版" Publication types
VSports注册入口 - MeSH terms
- Actions (V体育安卓版)
- V体育ios版 - Actions
- VSports最新版本 - Actions
- "VSports在线直播" Actions
"V体育2025版" Substances
- V体育ios版 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

