"VSports手机版" Adverse Outcome Pathways and Drug-Induced Liver Injury Testing
- PMID: 26119269
- PMCID: PMC4596002
- DOI: 10.1021/acs.chemrestox.5b00208
V体育2025版 - Adverse Outcome Pathways and Drug-Induced Liver Injury Testing
Abstract
Drug-induced liver injury is a prominent reason for premarketing and postmarketing drug withdrawal and can be manifested in a number of ways, such as cholestasis, steatosis, and fibrosis. The mechanisms driving these toxicological processes have been well characterized and have been emdedded in adverse outcome pathway frameworks in recent years. This review evaluates these constructs and simultaneously illustrates their use in the preclinical testing of drug-induced liver injury. VSports手机版.
V体育2025版 - Figures
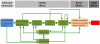
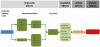
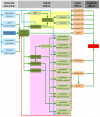
References
-
- Przybylak KR, Cronin MT. In silico models for drug-induced liver injury: current status. Expert Opin. Drug Metab. Toxicol. 2012;8:201–217. - PubMed
-
- Faa G, Ekstrom J, Castagnola M, Gibo Y, Ottonello G, Fanos V. A developmental approach to drug-induced liver injury in newborns and children. Curr. Med. Chem. 2012;19:4581–4594. - PubMed
-
- Verma S, Kaplowitz N. Diagnosis, management and prevention of drug-induced liver injury. Gut. 2009;58:1555–1564. - PubMed
-
- Andrade RJ, Agundez JA, Lucena MI, Martinez C, Cueto R, Garcia-Martin E. Pharmacogenomics in drug induced liver injury. Curr. Drug Metab. 2009;10:956–970. - PubMed
Publication types
"V体育ios版" MeSH terms
- Actions (VSports app下载)
- Actions (V体育平台登录)
- V体育2025版 - Actions
- "VSports在线直播" Actions
- V体育2025版 - Actions
- V体育官网 - Actions
Substances
- "V体育安卓版" Actions
- "V体育平台登录" Actions
- "VSports手机版" Actions
Grants and funding
"V体育平台登录" LinkOut - more resources
Full Text Sources
V体育2025版 - Other Literature Sources
Medical

