Engineering the gut microbiota to treat hyperammonemia
- PMID: 26098218
- PMCID: V体育2025版 - PMC4563680
- DOI: "V体育2025版" 10.1172/JCI79214
Engineering the gut microbiota to treat hyperammonemia
V体育官网 - Abstract
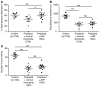
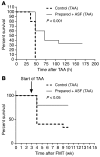
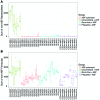
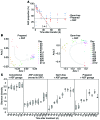
Increasing evidence indicates that the gut microbiota can be altered to ameliorate or prevent disease states, and engineering the gut microbiota to therapeutically modulate host metabolism is an emerging goal of microbiome research. In the intestine, bacterial urease converts host-derived urea to ammonia and carbon dioxide, contributing to hyperammonemia-associated neurotoxicity and encephalopathy in patients with liver disease. Here, we engineered murine gut microbiota to reduce urease activity VSports手机版. Animals were depleted of their preexisting gut microbiota and then inoculated with altered Schaedler flora (ASF), a defined consortium of 8 bacteria with minimal urease gene content. This protocol resulted in establishment of a persistent new community that promoted a long-term reduction in fecal urease activity and ammonia production. Moreover, in a murine model of hepatic injury, ASF transplantation was associated with decreased morbidity and mortality. These results provide proof of concept that inoculation of a prepared host with a defined gut microbiota can lead to durable metabolic changes with therapeutic utility. .
Figures


"VSports注册入口" References
-
- Backhed F, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12(5):611–622. doi: 10.1016/j.chom.2012.10.012. - "VSports注册入口" DOI - PubMed
"VSports" Publication types
- V体育平台登录 - Actions
"V体育平台登录" MeSH terms
- Actions (V体育2025版)
- V体育2025版 - Actions
- VSports最新版本 - Actions
- Actions (VSports app下载)
- Actions (V体育官网入口)
- V体育2025版 - Actions
- "VSports" Actions
- "VSports在线直播" Actions
- Actions (VSports手机版)
Substances
- V体育官网入口 - Actions
- "V体育2025版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

