"VSports" Programmed cell death 5 mediates HDAC3 decay to promote genotoxic stress response
- PMID: 26077467
- PMCID: PMC4490383
- DOI: 10.1038/ncomms8390
"VSports最新版本" Programmed cell death 5 mediates HDAC3 decay to promote genotoxic stress response
Erratum in
-
V体育平台登录 - Corrigendum: Programmed cell death 5 mediates HDAC3 decay to promote genotoxic stress response.Nat Commun. 2015 Aug 21;6:8225. doi: 10.1038/ncomms9225. Nat Commun. 2015. PMID: 26293611 Free PMC article. No abstract available.
"V体育平台登录" Abstract
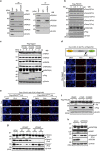
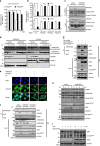
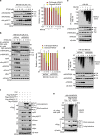
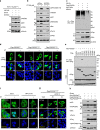
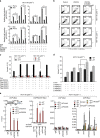
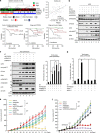
The inhibition of p53 activity by histone deacetylase 3 (HDAC3) has been reported, but the precise molecular mechanism is unknown. Here we show that programmed cell death 5 (PDCD5) selectively mediates HDAC3 dissociation from p53, which induces HDAC3 cleavage and ubiquitin-dependent proteasomal degradation. Casein kinase 2 alpha phosphorylates PDCD5 at Ser-119 to enhance its stability and importin 13-mediated nuclear translocation of PDCD5 VSports手机版. Genetic deletion of PDCD5 abrogates etoposide (ET)-induced p53 stabilization and HDAC3 cleavage, indicating an essential role of PDCD5 in p53 activation. Restoration of PDCD5(WT) in PDCD5(-/-) MEFs restores ET-induced HDAC3 cleavage. Reduction of both PDCD5 and p53, but not reduction of either protein alone, significantly enhances in vivo tumorigenicity of AGS gastric cancer cells and correlates with poor prognosis in gastric cancer patients. Our results define a mechanism for p53 activation via PDCD5-dependent HDAC3 decay under genotoxic stress conditions. .
"V体育ios版" Figures

References
-
- Vazquez A., Bond E. E., Levine A. J. & Bond G. L. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat. Rev. Drug Discov. 7, 979–987 (2008). - V体育2025版 - PubMed
-
- Vousden K. H. p53: death star. Cell 103, 691–694 (2000). - PubMed (VSports最新版本)
-
- Bode A. M. & Dong Z. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer 4, 793–805 (2004). - PubMed
-
- Kruse J. P. & Gu W. Modes of p53 regulation. Cell 137, 609–622 (2009). - "VSports手机版" PMC - PubMed
-
- Oliner J. D. et al.. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature 362, 857–860 (1993). - PubMed
Publication types
"V体育平台登录" MeSH terms
- V体育ios版 - Actions
- "VSports app下载" Actions
- Actions (V体育安卓版)
- V体育平台登录 - Actions
- V体育平台登录 - Actions
- V体育官网 - Actions
- "V体育官网入口" Actions
- "V体育2025版" Actions
- V体育2025版 - Actions
- "VSports手机版" Actions
- VSports在线直播 - Actions
- Actions (VSports最新版本)
- Actions (V体育平台登录)
- "VSports app下载" Actions
Substances
- "V体育官网入口" Actions
- Actions (V体育官网入口)
- "VSports app下载" Actions
- Actions (VSports在线直播)
- "V体育平台登录" Actions
- "VSports" Actions
LinkOut - more resources (VSports在线直播)
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases (VSports手机版)
VSports在线直播 - Research Materials
Miscellaneous

