Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation
- PMID: 25924064
- PMCID: PMC4498984
- DOI: V体育ios版 - 10.1038/nature14452
"VSports" Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation
Abstract
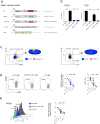
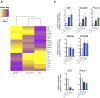
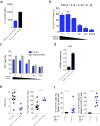
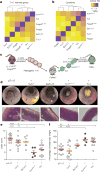
Inflammation is a beneficial host response to infection but can contribute to inflammatory disease if unregulated. The Th17 lineage of T helper (Th) cells can cause severe human inflammatory diseases. These cells exhibit both instability (they can cease to express their signature cytokine, IL-17A) and plasticity (they can start expressing cytokines typical of other lineages) upon in vitro re-stimulation. However, technical limitations have prevented the transcriptional profiling of pre- and post-conversion Th17 cells ex vivo during immune responses. Thus, it is unknown whether Th17 cell plasticity merely reflects change in expression of a few cytokines, or if Th17 cells physiologically undergo global genetic reprogramming driving their conversion from one T helper cell type to another, a process known as transdifferentiation. Furthermore, although Th17 cell instability/plasticity has been associated with pathogenicity, it is unknown whether this could present a therapeutic opportunity, whereby formerly pathogenic Th17 cells could adopt an anti-inflammatory fate. Here we used two new fate-mapping mouse models to track Th17 cells during immune responses to show that CD4(+) T cells that formerly expressed IL-17A go on to acquire an anti-inflammatory phenotype. The transdifferentiation of Th17 into regulatory T cells was illustrated by a change in their signature transcriptional profile and the acquisition of potent regulatory capacity. Comparisons of the transcriptional profiles of pre- and post-conversion Th17 cells also revealed a role for canonical TGF-β signalling and consequently for the aryl hydrocarbon receptor (AhR) in conversion. Thus, Th17 cells transdifferentiate into regulatory cells, and contribute to the resolution of inflammation. Our data suggest that Th17 cell instability and plasticity is a therapeutic opportunity for inflammatory diseases. VSports手机版.
Conflict of interest statement
Author Information The authors declare no competing financial interests V体育安卓版. Readers are welcome to comment on the online versionof the paper.
Figures (VSports手机版)








Comment in
-
T CELLS: Role reversal.Nat Rev Immunol. 2015 Jun;15(6):332. doi: 10.1038/nri3864. Nat Rev Immunol. 2015. PMID: 25998962 No abstract available.
References
-
- Hirota K, et al. FatemappingofIL-17-producing T cells in inflammatory responses. Nature Immunol. 2011;12:255–263. - "V体育2025版" PMC - PubMed
-
- Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. - PubMed (V体育官网入口)
-
- Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122:1180–1188. - PMC (V体育官网入口) - PubMed
Publication types (V体育官网)
MeSH terms
- VSports最新版本 - Actions
- "VSports注册入口" Actions
- V体育安卓版 - Actions
- VSports在线直播 - Actions
- Actions (V体育官网入口)
- Actions (V体育2025版)
- V体育ios版 - Actions
- VSports app下载 - Actions
Associated data
- "V体育安卓版" Actions
Grants and funding
"V体育ios版" LinkOut - more resources
"V体育安卓版" Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

