Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer
- PMID: 25897158
- PMCID: PMC4672027
- DOI: 10.1200/JCO.2014.58.3708
Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer
Abstract
Purpose: Programmed death 1 is an immune checkpoint that suppresses antitumor immunity. Nivolumab, a fully human immunoglobulin G4 programmed death 1 immune checkpoint inhibitor antibody, was active and generally well tolerated in patients with advanced solid tumors treated in a phase I trial with expansion cohorts VSports手机版. We report overall survival (OS), response durability, and long-term safety in patients with non-small-cell lung cancer (NSCLC) receiving nivolumab in this trial. .
Patients and methods: Patients (N = 129) with heavily pretreated advanced NSCLC received nivolumab 1, 3, or 10 mg/kg intravenously once every 2 weeks in 8-week cycles for up to 96 weeks. Tumor burden was assessed by RECIST (version 1. 0) after each cycle. V体育安卓版.
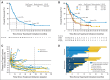
Results: Median OS across doses was 9. 9 months; 1-, 2-, and 3-year OS rates were 42%, 24%, and 18%, respectively, across doses and 56%, 42%, and 27%, respectively, at the 3-mg/kg dose (n = 37) chosen for further clinical development V体育ios版. Among 22 patients (17%) with objective responses, estimated median response duration was 17. 0 months. An additional six patients (5%) had unconventional immune-pattern responses. Response rates were similar in squamous and nonsquamous NSCLC. Eighteen responding patients discontinued nivolumab for reasons other than progressive disease; nine (50%) of those had responses lasting > 9 months after their last dose. Grade 3 to 4 treatment-related adverse events occurred in 14% of patients. Three treatment-related deaths (2% of patients) occurred, each associated with pneumonitis. .
Conclusion: Nivolumab monotherapy produced durable responses and encouraging survival rates in patients with heavily pretreated NSCLC VSports最新版本. Randomized clinical trials with nivolumab in advanced NSCLC are ongoing. .
© 2015 by American Society of Clinical Oncology V体育平台登录. .
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www VSports注册入口. jco. org. Author contributions are found at the end of this article.
V体育2025版 - Figures
"V体育平台登录" Comment in
-
Durable Responses With PD-1 Inhibition in Lung and Kidney Cancer and the Ongoing Search for Predictive Biomarkers.J Clin Oncol. 2015 Jun 20;33(18):1993-4. doi: 10.1200/JCO.2015.61.4172. Epub 2015 Apr 27. J Clin Oncol. 2015. PMID: 25918290 No abstract available.
"VSports注册入口" References
-
- Gettinger S, Lynch T. A decade of advances in treatment for advanced non-small cell lung cancer. Clin Chest Med. 2011;32:839–851. - PubMed (V体育2025版)
-
- Socinski MA, Evans T, Gettinger S, et al. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e341S–e368S. - PMC (VSports注册入口) - PubMed
-
- Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: A phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3342–3350. - PubMed
-
- Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:20–30. - PubMed
Publication types
- "V体育官网入口" Actions
MeSH terms (V体育安卓版)
- Actions (VSports注册入口)
- "VSports手机版" Actions
- VSports app下载 - Actions
- "VSports注册入口" Actions
- Actions (V体育ios版)
- VSports注册入口 - Actions
- VSports注册入口 - Actions
"VSports" Substances
- VSports手机版 - Actions
- Actions (VSports注册入口)
"V体育安卓版" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

