Rictor Undergoes Glycogen Synthase Kinase 3 (GSK3)-dependent, FBXW7-mediated Ubiquitination and Proteasomal Degradation
- PMID: 25897075
- PMCID: V体育ios版 - PMC4447982
- DOI: 10.1074/jbc.M114.633057
Rictor Undergoes Glycogen Synthase Kinase 3 (GSK3)-dependent, FBXW7-mediated Ubiquitination and Proteasomal Degradation
Abstract
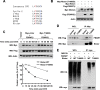
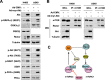
Rictor, an essential component of mTOR complex 2 (mTORC2), plays a pivotal role in regulating mTOR signaling and other biological functions. Posttranslational regulation of rictor (e. g. via degradation) and its underlying mechanism are largely undefined and thus are the focus of this study. Chemical inhibition of the proteasome increased rictor ubiquitination and levels. Consistently, inhibition of FBXW7 with various genetic means including knockdown, knock-out, and enforced expression of a dominant-negative mutant inhibited rictor ubiquitination and increased rictor levels, whereas enforced expression of FBXW7 decreased rictor stability and levels. Moreover, we detected an interaction between FBXW7 and rictor. Hence, rictor is degraded through an FBXW7-mediated ubiquitination/proteasome mechanism. We show that this process is dependent on glycogen synthase kinase 3 (GSK3): GSK3 was associated with rictor and directly phosphorylated the Thr-1695 site in a putative CDC4 phospho-degron motif of rictor; mutation of this site impaired the interaction between rictor and FBXW7, decreased rictor ubiquitination, and increased rictor stability VSports手机版. Finally, enforced activation of Akt enhanced rictor levels and increased mTORC2 activity as evidenced by increased formation of mTORC2 and elevated phosphorylation of Akt, SGK1, and PKCα. Hence we suggest that PI3K/Akt signaling may positively regulate mTORC2 signaling, likely through suppressing GSK3-dependent rictor degradation. .
Keywords: Akt PKB; FBXW7; GSK3; mTOR complex (mTORC); protein degradation; rictor; signal transduction; ubiquitylation (ubiquitination). V体育安卓版.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc V体育ios版. .
Figures






"V体育官网" References
-
- Guertin D. A., Stevens D. M., Saitoh M., Kinkel S., Crosby K., Sheen J. H., Mullholland D. J., Magnuson M. A., Wu H., Sabatini D. M. (2009) mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell 15, 148–159 - VSports app下载 - PMC - PubMed
-
- Roulin D., Cerantola Y., Dormond-Meuwly A., Demartines N., Dormond O. (2010) Targeting mTORC2 inhibits colon cancer cell proliferation in vitro and tumor formation in vivo. Mol Cancer 9, 57. - VSports app下载 - PMC - PubMed
-
- Gao D., Wan L., Inuzuka H., Berg A. H., Tseng A., Zhai B., Shaik S., Bennett E., Tron A. E., Gasser J. A., Lau A., Gygi S. P., Harper J. W., DeCaprio J. A., Toker A., Wei W. (2010) Rictor forms a complex with Cullin-1 to promote SGK1 ubiquitination and destruction. Mol. Cell 39, 797–808 - VSports注册入口 - PMC - PubMed
Publication types
MeSH terms
- V体育官网入口 - Actions
- "V体育官网" Actions
- "VSports" Actions
- VSports手机版 - Actions
- Actions (VSports最新版本)
- "VSports最新版本" Actions
- V体育ios版 - Actions
- "V体育安卓版" Actions
- "V体育2025版" Actions
- V体育平台登录 - Actions
- Actions (VSports手机版)
Substances
- "V体育官网" Actions
- "V体育安卓版" Actions
- Actions (V体育官网入口)
- "VSports在线直播" Actions
- Actions (VSports)
- "VSports app下载" Actions
- "VSports最新版本" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"V体育官网" Molecular Biology Databases
Research Materials
VSports手机版 - Miscellaneous

