"V体育官网" A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer
- PMID: 25890361
- PMCID: "VSports在线直播" PMC4438636
- DOI: "VSports在线直播" 10.1186/s12967-015-0460-x
VSports注册入口 - A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer
Abstract (V体育ios版)
Purpose: Recurrent platinum-resistant ovarian cancer has no curative options, necessitating the development of novel treatments, including immunotherapy VSports手机版. .
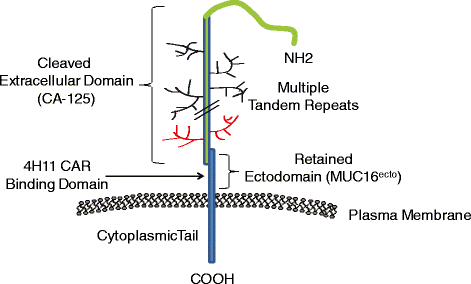
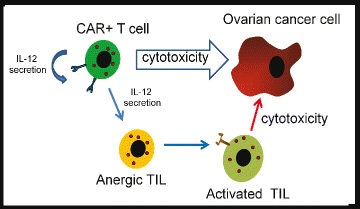
Rationale: Patient-derived T cells can be genetically modified to express chimeric antigen receptors (CARs) specific to tumor-associated antigens in an HLA-independent manner, with promising preclinical results. MUC16(ecto) is highly expressed on most epithelial ovarian carcinomas but at low levels on normal tissues, offering an excellent immunotherapeutic target for this cancer. CAR T cells further modified to secrete IL-12 show enhanced cytotoxicity, persistence, and modulation of the tumor microenvironment. V体育安卓版.
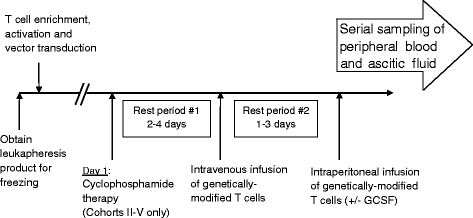
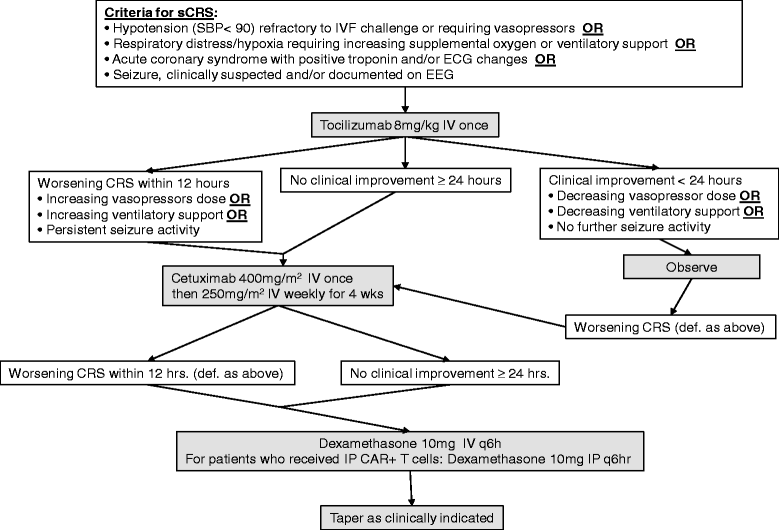
Design: We propose a dose escalation phase I clinical trial for patients with recurrent MUC-16(ecto+) ovarian cancer to test the safety of intravenous and intraperitoneal administration and the preliminary efficacy of autologous IL-12 secreting, MUC-16(ecto) CAR T cells containing a safety elimination gene. V体育ios版.
Innovation: This trial targets MUC-16(ecto), a novel and promising tumor-associated antigen. This will be the first time CAR T cells are injected intraperitoneally directly into the site of the tumor within the abdomen in humans VSports最新版本. Furthermore, the ability of genetically modified cells to secrete IL-12 will potentially enhance CAR T cell persistence and modulate the tumor microenvironment. For safety purposes, an elimination gene has been incorporated into the CAR T cells to mitigate any on-target, off-tumor or other unforeseen toxicity. .
Figures
"VSports在线直播" References
-
- du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–9. doi: 10.1093/jnci/djg036. - VSports - DOI - PubMed
-
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. - DOI - PubMed
Publication types
MeSH terms
- Actions (VSports注册入口)
- Actions (VSports app下载)
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- Actions (V体育安卓版)
- "V体育2025版" Actions
- Actions (V体育平台登录)
- VSports在线直播 - Actions
- "V体育安卓版" Actions
Substances
- Actions (V体育安卓版)
- Actions (V体育官网)
"V体育平台登录" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous