VSports app下载 - Metabolic reprogramming in triple-negative breast cancer through Myc suppression of TXNIP
- PMID: 25870263
- PMCID: PMC4418882
- DOI: V体育2025版 - 10.1073/pnas.1501555112
Metabolic reprogramming in triple-negative breast cancer through Myc suppression of TXNIP
Abstract
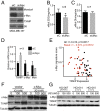
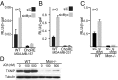
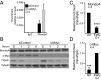
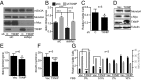
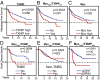
Triple-negative breast cancers (TNBCs) are aggressive and lack targeted therapies. Understanding how nutrients are used in TNBCs may provide new targets for therapeutic intervention VSports手机版. We demonstrate that the transcription factor c-Myc drives glucose metabolism in TNBC cells but does so by a previously unappreciated mechanism that involves direct repression of thioredoxin-interacting protein (TXNIP). TXNIP is a potent negative regulator of glucose uptake, aerobic glycolysis, and glycolytic gene expression; thus its repression by c-Myc provides an alternate route to c-Myc-driven glucose metabolism. c-Myc reduces TXNIP gene expression by binding to an E-box-containing region in the TXNIP promoter, possibly competing with the related transcription factor MondoA. TXNIP suppression increases glucose uptake and drives a dependence on glycolysis. Ectopic TXNIP expression decreases glucose uptake, reduces cell proliferation, and increases apoptosis. Supporting the biological significance of the reciprocal relationship between c-Myc and TXNIP, a Mychigh/TXNIPlow gene signature correlates with decreased overall survival and decreased metastasis-free survival in breast cancer. The correlation between the Mychigh/TXNIPlow gene signature and poor clinical outcome is evident only in TNBC, not in other breast cancer subclasses. Mutation of TP53, which is a defining molecular feature of TNBC, enhances the correlation between the Mychigh/TXNIPlow gene signature and death from breast cancer. Because Myc drives nutrient utilization and TXNIP restricts glucose availability, we propose that the Mychigh/TXNIPlow gene signature coordinates nutrient utilization with nutrient availability. Further, our data suggest that loss of the p53 tumor suppressor cooperates with Mychigh/TXNIPlow-driven metabolic dysregulation to drive the aggressive clinical behavior of TNBC. .
Keywords: MondoA; Myc; glycolysis; thioredoxin-interacting protein; triple-negative breast cancer V体育安卓版. .
"V体育官网入口" Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. - PubMed
-
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. - "V体育官网" PubMed
-
- Palaskas N, et al. 18F-fluorodeoxy-glucose positron emission tomography marks MYC-overexpressing human basal-like breast cancers. Cancer Res. 2011;71(15):5164–5174. - "V体育官网入口" PMC - PubMed
-
- Alles MC, et al. Meta-analysis and gene set enrichment relative to er status reveal elevated activity of MYC and E2F in the “basal” breast cancer subgroup. PLoS ONE. 2009;4(3):e4710. - PMC (VSports) - PubMed
V体育官网 - Publication types
MeSH terms
- "V体育官网入口" Actions
- "V体育ios版" Actions
- "V体育ios版" Actions
- "V体育安卓版" Actions
- "VSports" Actions
- VSports注册入口 - Actions
- V体育官网 - Actions
V体育2025版 - Substances
- Actions (V体育2025版)
- Actions (V体育安卓版)
- Actions (VSports最新版本)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
VSports注册入口 - Molecular Biology Databases
Research Materials
Miscellaneous

